NovaSeq 6000 Integration v3.2.1 Validation and Troubleshooting Guide
This guide explains how to validate the installation of the Illumina NovaSeq 6000 Integration Package v3.2.1.
The validation process involves:
|
•
|
Running samples through the Library Prep Validation v1.0 workflow. |
|
–
|
The workflow contains a single-step protocol that models the library prep required to produce normalized libraries. At the end of the step, the normalized libraries are advanced to the workflow selected by the user. |
|
•
|
Running normalized libraries through the NovaSeq 6000 v3.0 workflow. This validates the following: |
|
–
|
Successful routing of samples from the Run Format (NovaSeq 6000 v3.0) step to the NovaSeq Standard (NovaSeq 6000 v3.0) or NovaSeq Xp (NovaSeq 6000 v3.0) step. |
|
–
|
Automated generation of a sample sheet for use with bcl2fastq2 v2.20 analysis software. This file is automatically uploaded to the sequencing instrument via the Sequencer API and is used to set up the run. |
|
–
|
Automatic validation of run setup information. This information is uploaded to the NovaSeq Control Software (NVCS) via the the Sequencer API and is used to create the run recipe and initiate the run. |
|
–
|
Automated tracking of the NovaSeq sequencing run and parsing of run statistics into the LIMS, via the Sequencer API. |
Before You Begin
Before you begin to run the validation steps below, note that these steps assume that you have installed the Illumina NovaSeq 6000 Integration Package v3.2.1 and have imported the default LIMS configuration.
Validation Steps
Activate Workflow, Create Project, Add and Assign Samples
The following steps set up the LIMS in preparation for running samples through the Library Prep Validation v1.0 and NovaSeq 6000 v3.0 workflows.
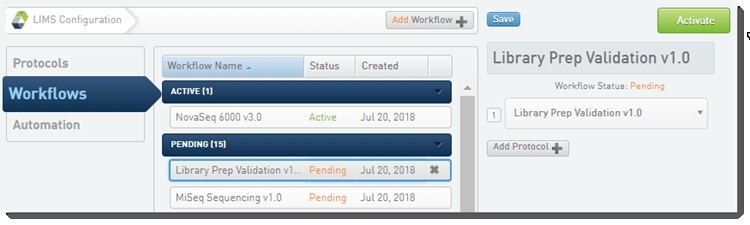
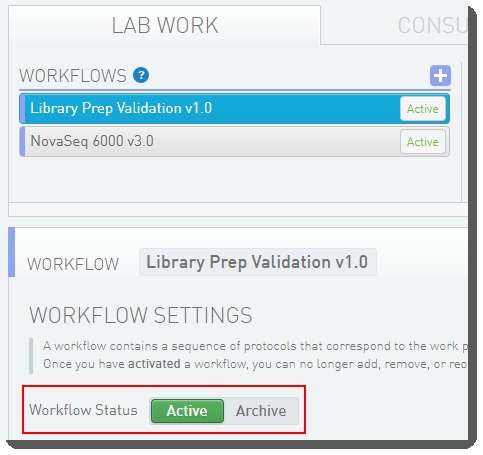
Step 1: Activate the Workflow
In the LIMS configuration area, activate the Library Prep Validation v1.0 and NovaSeq 6000 v3.0 workflows.
Workflow configuration and activation - LIMS v4.2:
Workflow configuration and activation - LIMS v5:
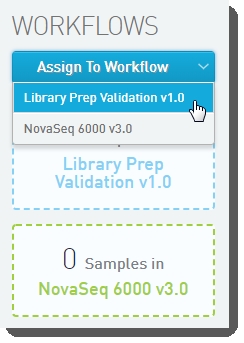
Step 2: Create a Project, Add Samples
|
1.
|
On the Projects and Samples screen, create a project and add samples to it. |
|
2.
|
Assign your samples to the Library Prep Validation v1.0 workflow. |
Library Prep Protocol: Library Prep Validation v1.0
This single-step protocol models the library prep required to produce normalized libraries that are ready for the NovaSeq 6000 v3.0 workflow.
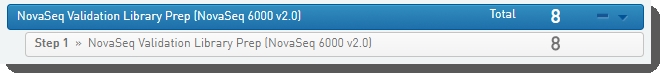
Step 1: Run Library Prep Validation v1.0
|
1.
|
In Lab View, locate the Library Prep Validation v1.0 protocol. You will see your samples queued for the Library Prep Validation v1.0 step. |
|
2.
|
Add the samples to the Ice Bucket and click View Ice Bucket. |
|
3.
|
On the Ice Bucket screen, click Begin Work. |
|
4.
|
On the Placement screen: |
|
•
|
In the Placed Samples area on the right, in the container field, scan or enter the barcode of the destination container. |
|
•
|
Select the samples from the Samples to be Placed area on the left, and then drag them over to the container wells on the right. |

|
5.
|
On the Add Labels screen, the Labels to Add list is populated with labels from the TruSeq HT Adapters v2 (D7-D5) label group. This is the only label group configured in the out-of-the-box integration and it is used by default in this step. |
|
•
|
Select labels on the left, and drag them over to the container on the right to assign them to placed samples. |
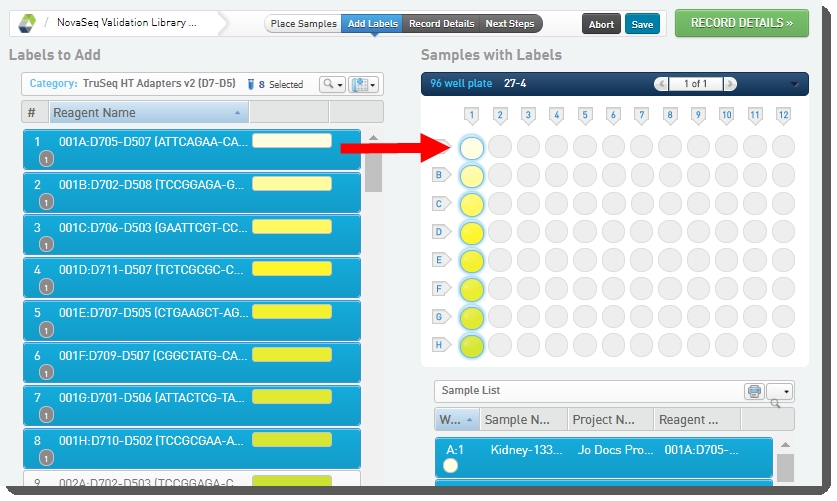
|
6.
|
On the Record Details screen: |
|
•
|
In the Sample Details table, enter values for Normalized Molarity. You can type values individually, or use Copy/Paste functionality to enter multiple values from a spreadsheet. |
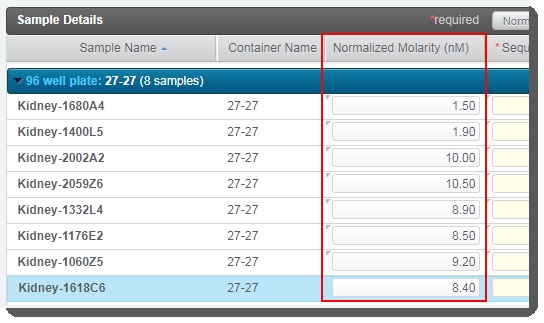
|
•
|
The Sequencing Instrument column indicates the instrument to be used for the sequencing run. Select NovaSeq 3.0. |
You can populate the fields in this column using one of the following methods:
|
–
|
To populate fields individually for each sample: Select from the drop-down list in each row. |
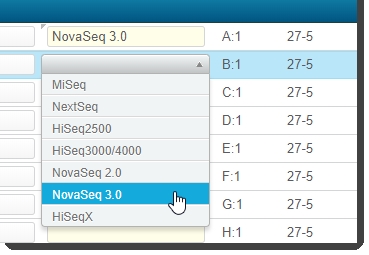
|
–
|
To populate fields all at once: Use Copy/Paste to enter multiple values from a spreadsheet. |
Alternatively, click inside the table and press Ctrl + A to select all rows. At the top of the table, select Sequencing Instrument and then select NovaSeq 3.0 from the adjacent drop-down list. Click Apply.
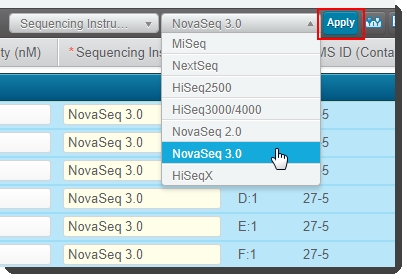
If you are working with a large number of samples, you can use Copy/Paste functionality to enter multiple values from a spreadsheet.
|
a.
|
Click the Copy Samples button to copy the sample information from the LIMS to the Clipboard. |
|
b.
|
Open a blank Excel document and paste the contents of the Clipboard into the spreadsheet. |
|
c.
|
In the spreadsheet, enter the Normalized Molarity and Sequencing Instrument values. Select the entire spreadsheet, including the headers (Ctrl + A), and copy (Ctrl + C) the information to the Clipboard. |
|
d.
|
In the LIMS, click the Paste Samples button. Paste the contents of the clipboard (Ctrl + V) into the Update Table window and then click Update to paste into the Sample Details table. |
|
•
|
This triggers the Next Steps automation, which sets the value of the next step (for all samples) to Remove from workflow. Note that the Routing Script automation expects this value and requires it in order to successfully advance samples to the next step. |
|
8.
|
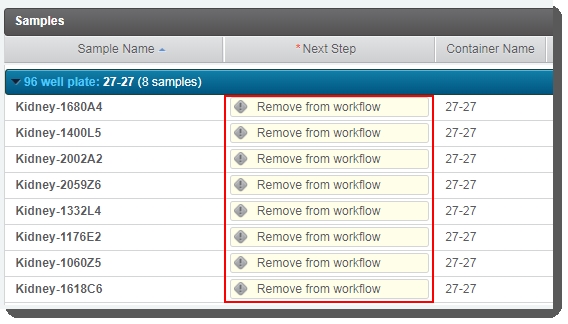
On the Assign Next Steps screen, in the Samples table, the Next Step for all samples is pre-populated with Remove from workflow. |
Do not change this value! If Next Step is not set to Remove from workflow, the routing script will not be able to route samples correctly.
On exit from the step, the Routing Script automation is triggered. This automation assigns samples to the first step of the NovaSeq 6000 v3.0 workflow - Define Run Format (NovaSeq 6000 v3.0). This is the only step in Protocol 1: Run Format (NovaSeq 6000 v3.0).
Protocol 1: Run Format (NovaSeq 6000 v3.0)
This protocol includes a single step - Define Run Format (NovaSeq 6000 v3.0). The step allows for the assignment of per sample values for Loading Workflow Type, Normalized Molarity, Flowcell Type, and Final Loading Concentration (pM). At the end of the step, samples are routed to the NovaSeq Standard or NovaSeq Xp protocol, according to the selected Loading Workflow Type.
Step 1: Run Define Run Format (NovaSeq 6000 v3.0)
|
1.
|
In Lab View, locate the Run Format (NovaSeq 6000 v3.0) protocol. You will see your samples queued for the Define Run Format (NovaSeq 6000 v3.0) step. |
|
2.
|
Add the samples to the Ice Bucket and click View Ice Bucket. |
|
3.
|
On the Ice Bucket screen, click Begin Work. |
|
4.
|
On the Record Details screen, in the Sample Details table, populate the following fields (values can vary across samples): |
|
•
|
Loading Workflow Type - Select NovaSeq Standard or NovaSeq Xp from the drop-down list. |
|
•
|
Flowcell Type - Select SP, S1, S2, or S4. |
|
•
|
Final Loading Concentration (pM) - You may select from the two preset options - 225 (for PCR-free workflows) or 400 (for Nano workflows), or you may enter a different value. |

|
•
|
The Normalized Molarity (nM) values are copied over from the previous step. If you did not populate this column during library prep, you can enter the values here. |
|
5.
|
Click Next Steps. This triggers the Next Steps automation, which: |
|
•
|
Sets the value of the next step (for all samples) to Remove from workflow. The Routing Script automation expects this value, and requires it in order to successfully advance samples to the next step. |
|
•
|
Calculates the Minimum Molarity. |
|
•
|
Checks Normalized Molarity value. For samples with no Normalized Molarity value (i.e., empty value, not including 0), generates an error message informing the user that the field cannot be empty. |
|
•
|
Compares each sample's Normalized Molarity value with the Minimum Molarity value. |
|
6.
|
On the Assign Next Steps screen: |
|
•
|
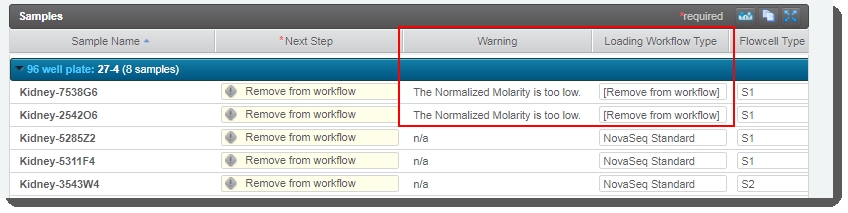
In the Sample Details table, the Next Step for all samples is pre-populated with Remove from workflow - regardless of the Loading Workflow Type. |
Do not change this value! If Next Step is not set to Remove from workflow, the routing script will not be able to route samples correctly.
|
•
|
For samples whose Normalized Molarity value was found to be lower than the Minimum Molarity value, the Loading Workflow Type is set to Remove from workflow and a message is recorded in the Warning field for the sample. |
At this point you have two options:
|
–
|
Return to the Record Details screen and adjust the Normalized Molarity value so that it equals or exceeds the Minimum Molarity value. You will also need to set the Loading Workflow Type to NovaSeq Standard or NovaSeq Xp, as applicable. |
|
–
|
Complete the protocol without correcting the Normalized Molarity value. In this case, the samples in question will be removed from the LIMS workflow. |
|
7.
|
Click Finish Step. The Routing Script automation is triggered: |
|
•
|
Samples whose Loading Workflow Type is set to Remove from workflow (i.e., where Normalized Molarity value is lower than the Minimum Molarity) are removed from the LIMS workflow. |
|
•
|
Samples whose Loading Workflow Type is NovaSeq Standard are routed to the Make Bulk Pool for NovaSeq Standard (NovaSeq 6000 v3.0) step. This is the first of two steps in Protocol 2: NovaSeq Standard (NovaSeq 6000 v3.0). |
|
•
|
Samples whose Loading Workflow Type is set to NovaSeq Xp are routed to the Make Bulk Pool for NovaSeq Xp (NovaSeq 6000 v3.0) step. This is the first of three steps in Protocol 3: NovaSeq Xp (NovaSeq 6000 v3.0). |
Protocol 2: NovaSeq Standard (NovaSeq 6000 v3.0)
In this protocol, samples are pooled and added to the library tube in preparation for the NovaSeq run. The protocol contains two steps:
|
1.
|
Make Bulk Pool for NovaSeq Standard (NovaSeq 6000 v3.0)
|
|
2.
|
Dilute and Denature (NovaSeq 6000 v3.0)
|
Step 1: Run Make Bulk Pool for NovaSeq Standard (NovaSeq 6000 v3.0)
|
1.
|
In Lab View, locate the NovaSeq Standard (NovaSeq 6000 v3.0) protocol. You will see your samples queued for the Make Bulk Pool for NovaSeq Standard (NovaSeq 6000 v3.0) step. |
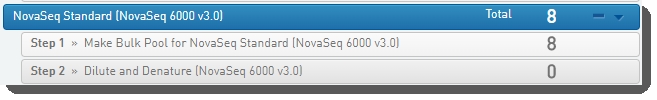
|
2.
|
On the Queue screen, add samples of the same Flowcell Type to the Ice Bucket and click View Ice Bucket. |
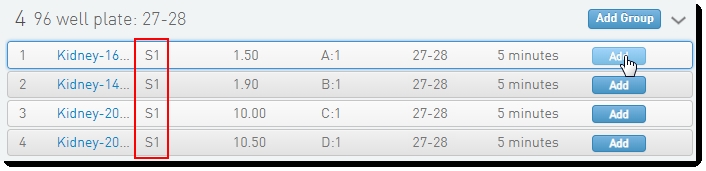
|
3.
|
On the Ice Bucket screen, click Begin Work. |
|
4.
|
On the Pooling screen: |
|
•
|
Create a pool by dragging samples into the Pool Creator. |
You must only create one pool.
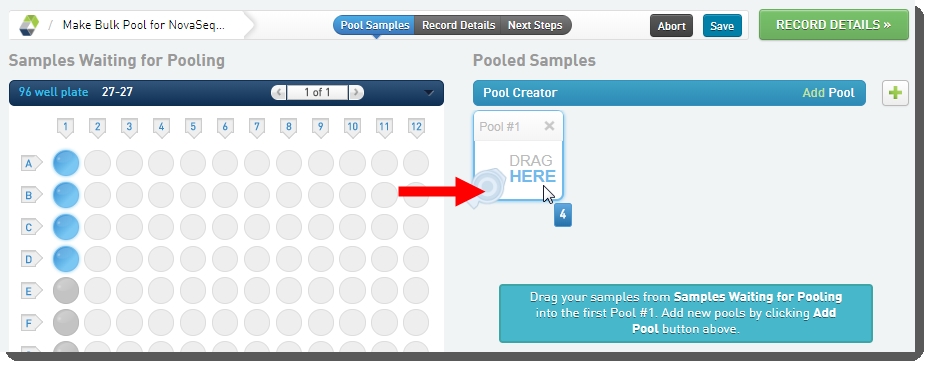
|
•
|
You can type a name for the pool, or accept the default name - Pool #1. |
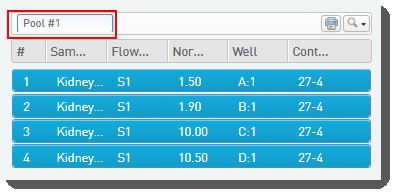
|
5.
|
On exit from the Pooling screen, the Validate Inputs Flowcell Type and Single Pool is triggered. The automation checks that: |
|
•
|
All samples in the pool have been assigned the same Flowcell Type. |
|
•
|
Only one pool has been created. |
|
6.
|
On the Record Details screen, the Step Details area contains two required fields: |
|
•
|
Number of Flowcells to Sequence - The value entered in this field is used in volume calculations, to ensure that volumes are sufficient for the number of times the pool will be sequenced. |
|
•
|
Minimum Per Sample Volume (ul) - The value in this field is used to calculate how much of each sample will be included in the pool. The field is pre-populated with the configured default value - 5 ul, but may be edited if required. |
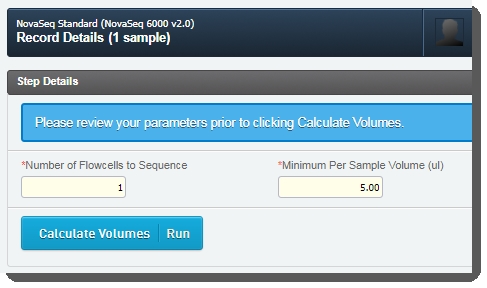
Assuming the default Minimum Per Sample Volume (ul) value of 5, for a given batch:
|
a.
|
If the smallest Per Sample Volume (ul) value is less than 5, the LIMS automatically assigns a value of 5 to the sample's Adjusted Per Sample Volume (ul) field. |
|
b.
|
The LIMS then adjusts the Adjusted Per Sample Volume (ul) field value for all other samples in the batch, based on the ratio used to increase the lowest value to 5. |
|
•
|
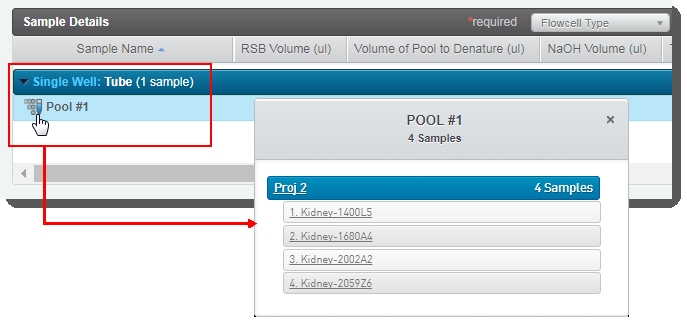
In the Sample Details table, you can click on the pool icon to view details on the pool composition. |
|
•
|
Click Calculate Volumes. This triggers the Calculate Volumes automation, which performs volume calculations based on the selected Flowcell Type: |
|
–
|
Calculates Bulk Pool Volume (ul) value. |
|
–
|
Sets the value of the Bulk Pool Volume (ul) field, based on the selected Flowcell Type. |
|
–
|
Calculates the Per Sample Volume (ul) value to be added to the pool. |
|
–
|
Calculates the Total Sample Volume (ul) value. |
|
–
|
If the Total Sample Volume is less than the Bulk Pool Volume, calculates the RSB Volume (ul) value. |
|
–
|
Populates the Flowcell Type and Loading Workflow Type columns of the Sample Details table. |
|
–
|
Populates the Volume of Pool to Denature (ul), NaOH Volume (ul) and Tris-HCI Volume (ul) columns of the Sample Details table. Values are set by a script and are not editable by the user. |
|
–
|
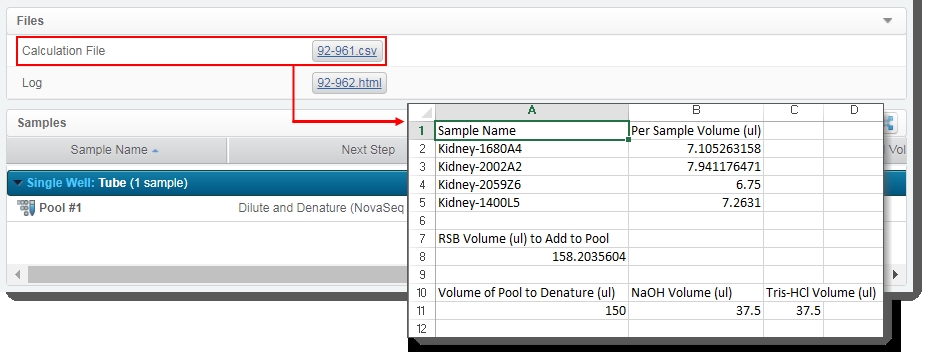
The automation also generates the Calculation File (CSV) and attaches it to the step. This file contains volume information about the pool and the individual samples it contains. Click on the file to download it and then open it in Excel. |
|
•
|
Click Next Steps. This triggers the Set Next Step automation. This automation sets the next step for samples to ADVANCE, advancing them to the next step in the protocol - Dilute and Denature (NovaSeq 6000 v3.0). |
|
7.
|
On the Assign Next Steps screen, the next step for samples is already set to the next step in the workflow: Dilute and Denature (NovaSeq 6000 v3.0). |
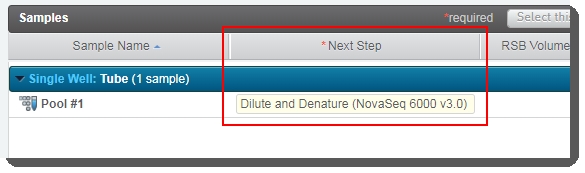
At the end of this step, the pool of samples automatically advances to the Dilute and Denature (NovaSeq 6000 v3.0) step.
Step 2: Run Dilute and Denature (NovaSeq 6000 v3.0)
|
1.
|
In Lab View, locate the NovaSeq Standard (NovaSeq 6000 v3.0) protocol. You will see the pool of samples queued for the Dilute and Denature (NovaSeq 6000 v3.0) step. |
|
2.
|
Add the samples to the Ice Bucket and click View Ice Bucket. |
|
3.
|
On the Ice Bucket screen, click Begin Work. The Validate Single Input automation is triggered. This automation checks that there is only one container input to the step. |
|
4.
|
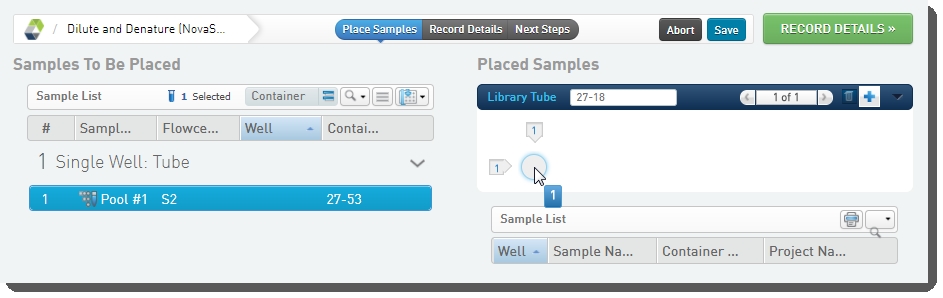
On the Placement screen: |
|
•
|
Drag the pool into the library tube in the Placed Samples area. |
|
•
|
Scan or type the barcode of the library tube into the Library Tubefield. |
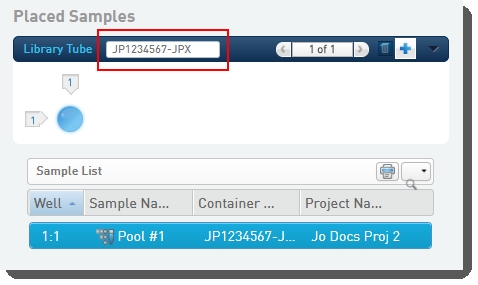
On exit of the Placement screen, the Validate Library Tube Barcode automation checks that the library tube barcode conforms to the barcode mask [A-Za-z]{2}[0-9]{7}-[A-Za-z]{3}, and displays an error message if this is not the case. This automation also copies the Flowcell Type and Loading Workflow Type values from step inputs to outputs.
|
5.
|
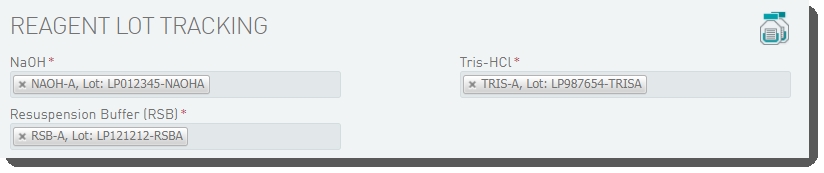
On the Record Details screen, the Reagent Lot Tracking section lets you track the NaOH, Resuspension Buffer, and Tris-HCI reagents used in the step. You will need to add and activate lots for these reagents. |
To add and activate reagent lots:
|
•
|
In a new browser tab, open an additional instance of the LIMS. LIMS v5.0: |
|
–
|
Navigate to the Consumables configuration screen. |
|
–
|
LIMS v4.2: Navigate to the Reagents and Controls screen. |
|
•
|
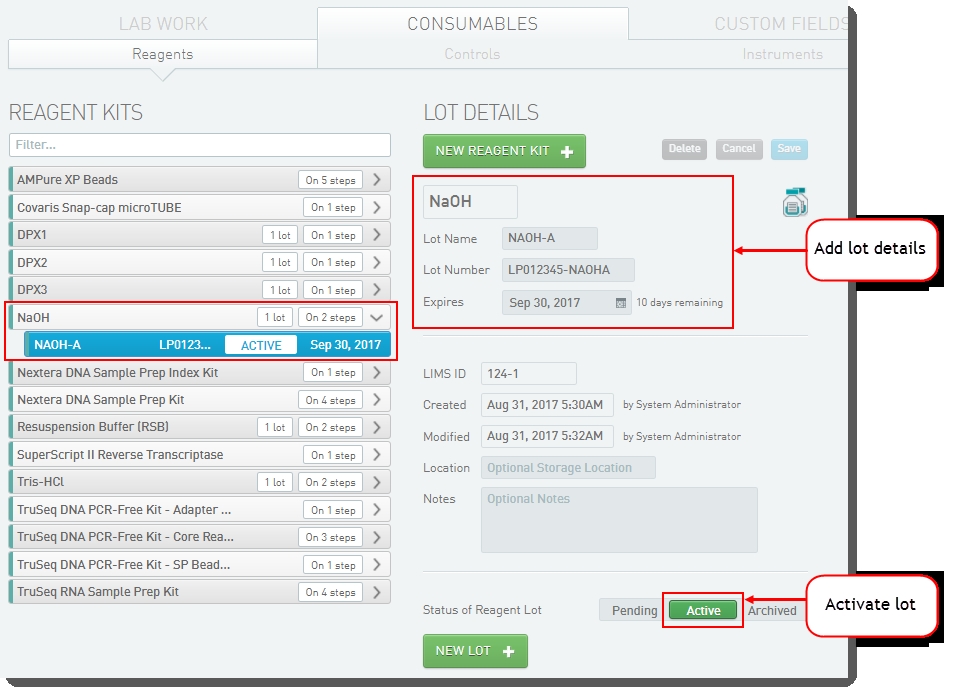
Select the NaOH reagent kit and click New Lot. |
|
•
|
Enter the lot details and click Save. |
|
•
|
Set the Status of Reagent Lot toggle switch to Active. |
|
•
|
Repeat this process to add and activate Resuspension Buffer and Tris-HCI reagent lots. |
|
•
|
Return to your original working browser tab and refresh the page. |
|
6.
|
In the Reagent Lot Tracking section, select from the active lots displayed in each drop-down list. |
|
7.
|
The fields displayed on the Record Details screen are used to set up the run and generate the sample sheet. |
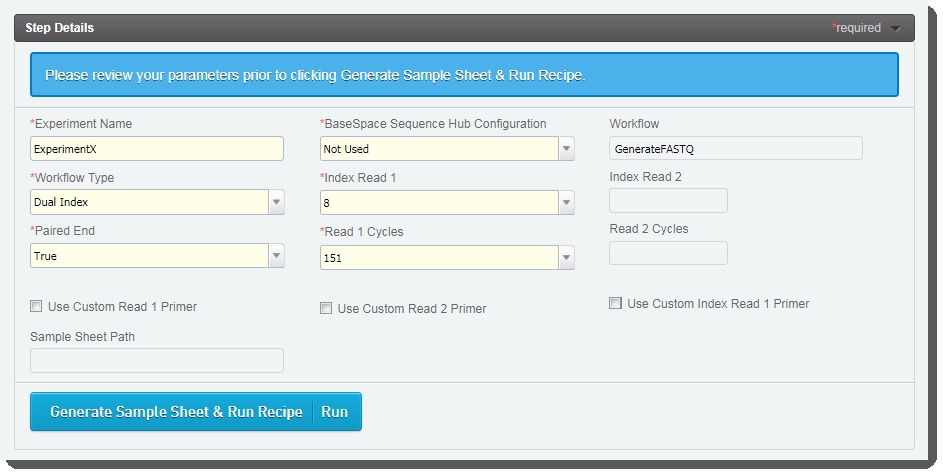
Some of these fields are auto-populated and some must be completed by the user (see the following table for details):
|
Field
|
Value
|
|
Experiment Name
|
Enter the experiment name. Only alphanumeric characters, dashes, and underscores are permitted. No spaces.
|
|
BaseSpace Sequence Hub Configuration
|
Select from preset options: Not Used, Run Monitoring Only, or Run Monitoring and Storage
|
|
Workflow
|
Automatically populated with preset - GenerateFASTQ
|
|
Workflow Type
|
Select from preset options: No Index, Single Index, Dual Index, or Custom
|
|
Index Read 1
|
Select from preset options: 0, 6, or 8 - or type a value between 0 and 20.
|
|
Index Read 2
|
Select from preset options: 0, 6, or 8 - or type a value between 0 and 20.
|
|
Paired End
|
Select from preset options: True or False
|
|
Read 1 Cycles
|
Select from preset options: 251, 151, 101, or 51 - or type a value between 1 and 251.
*Value of 251 is only supported for SP flow cell type. For all other flow cell types, maximum value is 151.
|
|
Read 2 Cycles
|
Select from preset options: 251, 151, 101, or 51 - or type a value between 1 and 251.
*Value of 251 is only supported for SP flow cell type. For all other flow cell types, maximum value is 151.
|
|
Use Custom Read 1 Primer
|
Select if applicable.
LIMS v5.0.5 and later: See Configuration Update for NovaSeq Integration with Clarity LIMS v5.0.5 and Later documentation.
|
|
Use Custom Read 2 Primer
|
Select if applicable.
LIMS v5.0.5 and later: See Configuration Update for NovaSeq Integration with Clarity LIMS v5.0.5 and Later documentation.
|
|
Use Custom Index Read 1 Primer
|
Select if applicable.
LIMS v5.0.5 and later: See Configuration Update for NovaSeq Integration with Clarity LIMS v5.0.5 and Later documentation.
|
|
Output Folder
|
Enter network path for sequencing run folder. For example:
\\networkshare\run_data
|
|
Use Custom Recipe
|
Select if applicable.
|
|
Custom Recipe Path
|
If you selected the Use Custom Recipe option, enter the path to the custom recipe file to be used.
|
|
8.
|
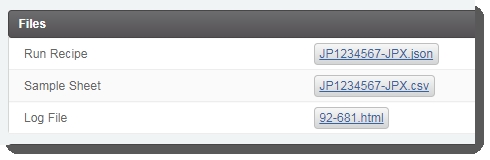
On the Record Details screen, click Validate Run Setup and Generate Sample Sheet . This triggers the automation script, which: |
|
•
|
Validates the parameters entered on the Record Details screen. |
|
•
|
Generates the sample sheet and attaches it to the placeholder in the Files area of the Record Detailsscreen. |
|
9.
|
On the Assign Next Steps screen, the Next Step for samples is pre-populated with Remove from workflow. The Routing Script automation expects this value, and requires it in order to successfully advance samples to the next step. |
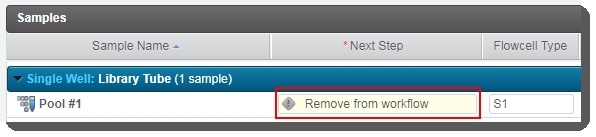
Do not change this value. If Next Step is not set to Remove from workflow, the routing script will not be able to route samples correctly.
On exit from the step:
|
•
|
The Routing Script automation is triggered and samples are routed to Protocol 4: AUTOMATED - NovaSeq Run (NovaSeq 6000 v3.0). |
|
•
|
In Lab View, the pool of samples is queued for the AUTOMATED - NovaSeq Run (NovaSeq 6000 v3.0) step. |
At this point in the workflow, the user interaction ends. Proceed to Protocol 4: AUTOMATED - NovaSeq Run (NovaSeq 6000 v3.0)
Do not add samples to the Ice Bucket or start the AUTOMATED - NovaSeq Run (NovaSeq 6000 v3.0) step. The integration will do this automatically.
Protocol 3: NovaSeq Xp (NovaSeq 6000 v3.0)
In this protocol, samples are pooled and added to lanes on the NovaSeq flow cell. The flow cell type is determined by the option selected in the Define Run Format (NovaSeq 6000 v3.0) step.
The protocol contains three steps:
|
1.
|
Make Bulk Pool for NovaSeq Xp (NovaSeq 6000 v3.0)
|
|
2.
|
Dilute, Denature and ExAmp (NovaSeq 6000 v3.0)
|
|
3.
|
Load to Flowcell (NovaSeq 6000 v3.0)
|
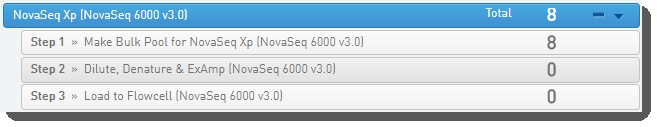
Step 1: Run Make Bulk Pool for NovaSeq Xp (NovaSeq 6000 v3.0)
|
1.
|
In Lab View, locate the NovaSeq Standard (NovaSeq 6000 v3.0) protocol. You will see your samples queued for the Make Bulk Pool for NovaSeq XP (NovaSeq 6000 v3.0) step. |
|
2.
|
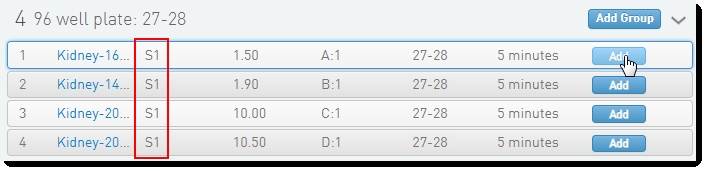
On the Queue screen, add samples of the same Flowcell Type to the Ice Bucket and click View Ice Bucket. |
|
3.
|
On the Ice Bucket screen, click Begin Work. |
|
4.
|
On the Pooling screen: |
|
•
|
Create a pool by dragging samples into the Pool Creator. |
You must only create one pool.

|
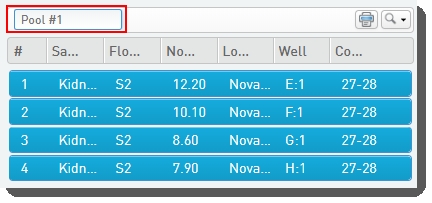
•
|
You can type a name for the pool, or accept the default name - Pool #1. |
|
5.
|
On exit from the Pooling screen, the Validate Inputs Flowcell Type and Single Pool is triggered. The automation checks that: |
|
•
|
All samples in the pool have been assigned the same Flowcell Type. |
|
•
|
Only one pool has been created |
|
6.
|
On the Record Details screen, the Step Details area contains two required fields: |
|
•
|
Number of Lanes to Sequence - The value entered in this field is used in volume calculations, to ensure that volumes are sufficient for the number of times the pool will be sequenced. |
|
•
|
Minimum Per Sample Volume (ul) - The value in this field is used to calculate how much of each sample will be included in the pool. The field is pre-populated with the configured default value - 5 ul, but may be edited if required. |
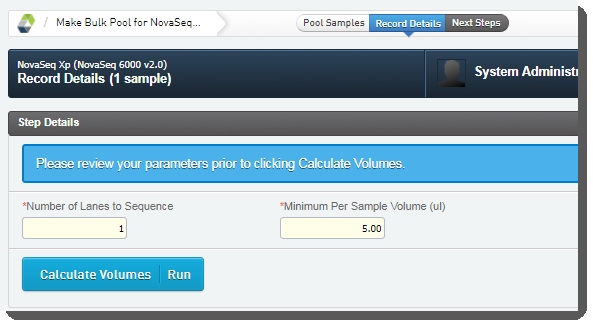
Assuming the default Minimum Per Sample Volume (ul) value of 5, for a given batch:
|
a.
|
If the smallest Per Sample Volume (ul) value is less than 5, the LIMS automatically assigns a value of 5 to the sample's Adjusted Per Sample Volume (ul) field. |
|
b.
|
The LIMS then adjusts the Adjusted Per Sample Volume (ul) field value for all other samples in the batch, based on the ratio used to increase the lowest value to 5. |
|
•
|
In the Sample Details table, you can click on the pool icon to view details on the pool composition. |

|
•
|
Click Calculate Volumes. This triggers the Calculate Volumes automation, which performs volume calculations based on the selected Flowcell Type: |
|
–
|
Calculates Bulk Pool Volume (ul) value. |
|
–
|
Calculates the Per Sample Volume (ul) value to be added to the pool. |
|
–
|
Calculates the Total Sample Volume (ul) value. |
|
–
|
If the Total Sample Volume is less than the Bulk Pool Volume, calculates the RSB Volume (ul) value. |
|
–
|
The automation also populates the Flowcell Type and Loading Workflow Type columns of the Sample Details table. |
|
–
|
The automation also generates the Calculation File (CSV) and attaches it to the step. This file contains volume information about the samples and RSB buffer to add to the pool. Click on the file to download it and then open it in Excel. |
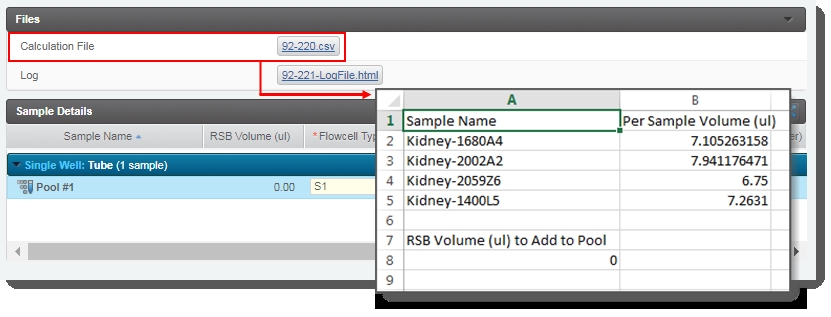
|
•
|
Click Next Steps. This triggers the Set Next Step automation, which: |
|
–
|
Copies the Flowcell Type values from the step inputs to the step outputs. |
|
–
|
Sets the next step for samples to ADVANCE, advancing them to the next step in the protocol - Dilute, Denature and ExAmp (NovaSeq 6000 v3.0). |
|
7.
|
On the Assign Next Steps screen, the next step for samples is already set to the next step in the workflow - Dilute, Denature and ExAmp (NovaSeq 6000 v3.0). |
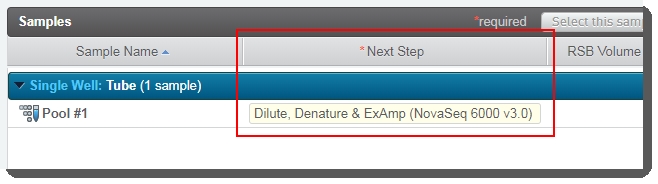
At the end of this step, the pool of samples automatically advances to the Dilute, Denature and ExAmp (NovaSeq 6000 v3.0) step.
Step 2: Run Dilute, Denature and ExAmp (NovaSeq 6000 v3.0)
|
1.
|
In Lab View, locate the NovaSeq Xp (NovaSeq 6000 v3.0) protocol. You will see the pool of samples queued for the Dilute, Denature and ExAmp (NovaSeq 6000 v3.0) step. |
|
2.
|
Add the pool to the Ice Bucket and click View Ice Bucket. |
|
3.
|
[Optional] On the Ice Bucket screen, set the number of derivatives to create (these will be placed into the flow cell lanes) and click Begin Work. |
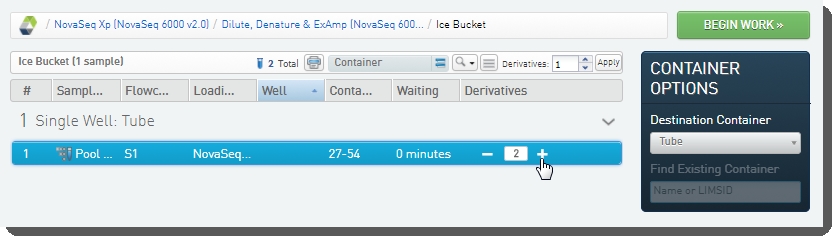
|
4.
|
On entry to the Record Details screen, the Calculate Volumes automation is triggered. This automation sets the following values based on the selected Flowcell Type: |
|
•
|
Mastermix per Lane (ul)
|
|
•
|
The automation also populates the Flowcell Type and Loading Workflow Type columns of the Sample Details table. |
|
•
|
The automation also generates the Calculation File (CSV) and attaches it to the step. This file contains information about the DPX Mastermix volume, and the volume of Mastermix, NaOH. and Tris-HCI to add per working pool (see the next step). |
|
5.
|
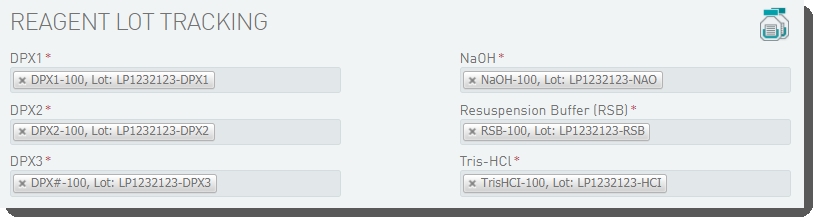
On the Record Details screen, the Reagent Lot Tracking section lets you track the DPX1, DPX2, DPX3, NaOH, Resuspension Buffer, and Tris-HCI reagents used in the step. You will need to add and activate lots for these reagents |
To add and activate reagent lots:
|
•
|
In a new browser tab, open an additional instance of the LIMS. LIMS v5.0: |
|
–
|
Navigate to the Consumables configuration screen.LIMS v4.2: |
|
–
|
Navigate to the Reagents and Controls screen. |
|
•
|
Select one of the reagent kits listed above (NaOH shown below) and click New Lot. |
|
•
|
Enter the lot details and click Save. |
|
•
|
Set the Status of Reagent Lot toggle switch to Active. |

|
•
|
Repeat this process to add and activate the remaining reagent lots. |
|
•
|
Return to your original working browser tab and refresh the page. |
|
6.
|
On the Record Details screen: |
|
•
|
In the Reagent Lot Tracking section, select from the active lots displayed in each drop-down list. |
|
•
|
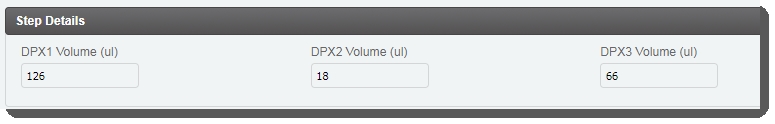
In the Step Details area, the DPX1, DPX2, and DPX3 reagent volume values are already populated. These values are set by a script and are not editable by the user running the step. |
|
•
|
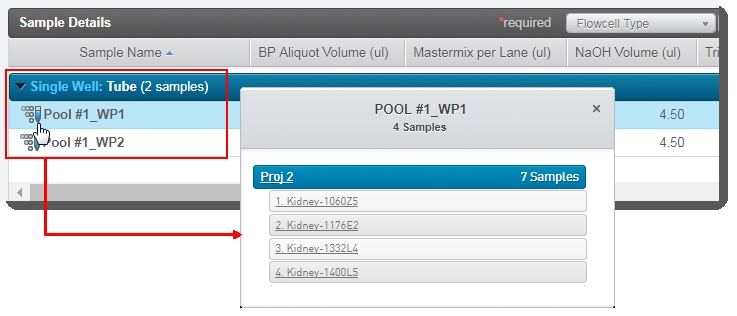
In the Sample Details table, you can click on the pool icon to view details on the working pool composition. |
|
–
|
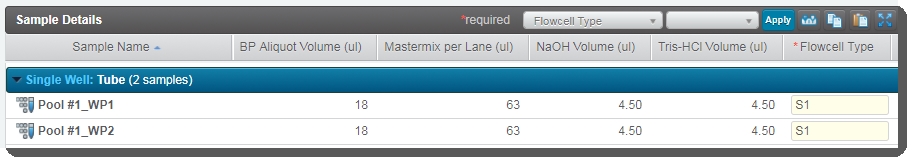
The BP Aliquot, Mastermix per lane, NaOH, and Tris-HCI volume values for each working pool are already populated. These values are set by a script and are not editable by the user running the step. |
|
–
|
The Flowcell Type and Loading Workflow Type columns are already populated. |
|
–
|
The working pool number is appended to the bulk pool name. This allows you to quickly identify which working pools are derived from the same bulk pool. |
| 7. | In the Files area, click on the Calculation File (CSV) to open it and view details on the DPX Mastermix volume, and the volume of Mastermix, NaOH. and Tris-HCI to add per working pool. |
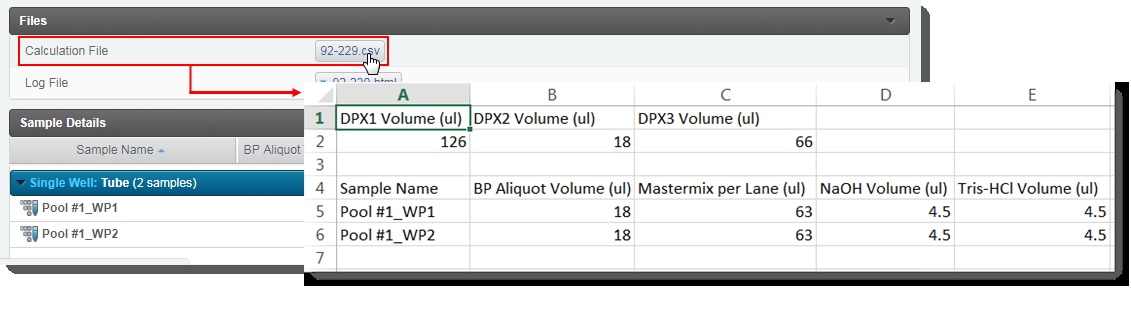
| 9. | On the Assign Next Steps screen, the next step is already set to Load to Flowcell (NovaSeq 6000 v3.0). |
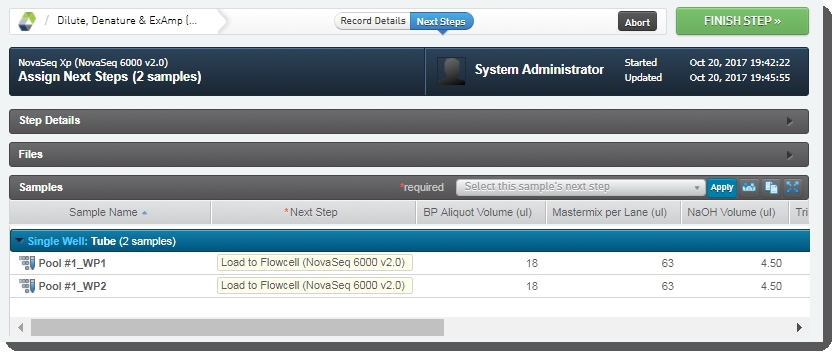
Step 3: Run Load to Flowcell (NovaSeq 6000 v3.0)
| 1. | On the Ice Bucket screen: |
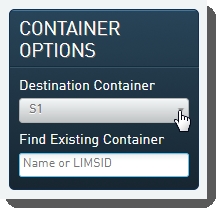
| • | In the Container Options panel, select the appropriate flow cell type from the Destination Containerdrop-down list. |
| 2. | The Validate Inputs and Selected Container automation checks that: |
| • | The Flowcell Type field has been set to a valid value (SP, S1, S2, or S4), and that all inputs have the same value for this field. |
| • | The container type selected matches the value in the Flowcell Type field. |
| • | The number of outputs matches the number of lanes on the selected flow cell type. If validation fails, an error message informs the user that the number of working pools does not match the number of lanes available on the flow cell. |
| 3. | On the Placement screen: |
| • | Drag the pool(s) from the left of the screen over into the Placed Samples area on the right. |
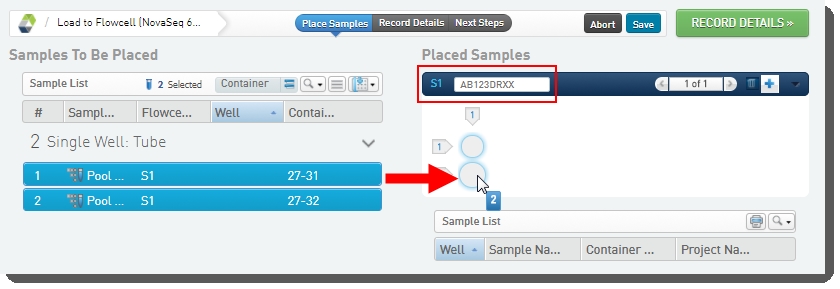
| • | Scan or type the barcode of the flow cell into the Flow Cell field. |
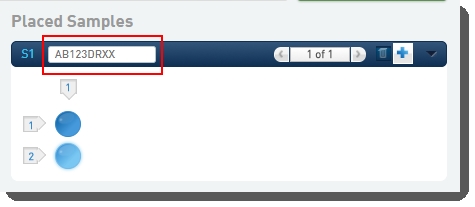
On exit of the Placement screen, the Validate Flowcell Barcode automation checks that the container barcode conforms to the barcode mask for the selected Flowcell Type, as listed in the following table. This automation also copies the Flowcell Type and Loading Workflow Type field values from step inputs to outputs.
Flow Cell Type
| Barcode Mask
|
SP | [A-Za-z0-9]{5}DRXX |
S1 | [A-Za-z0-9]{5}DRXX |
S2 | [A-Za-z0-9]{5}DMXX |
S4 | [A-Za-z0-9]{5}DSXX |
| 4. | The fields displayed on the Record Details screen are used to set up the run and generate the sample sheet. |
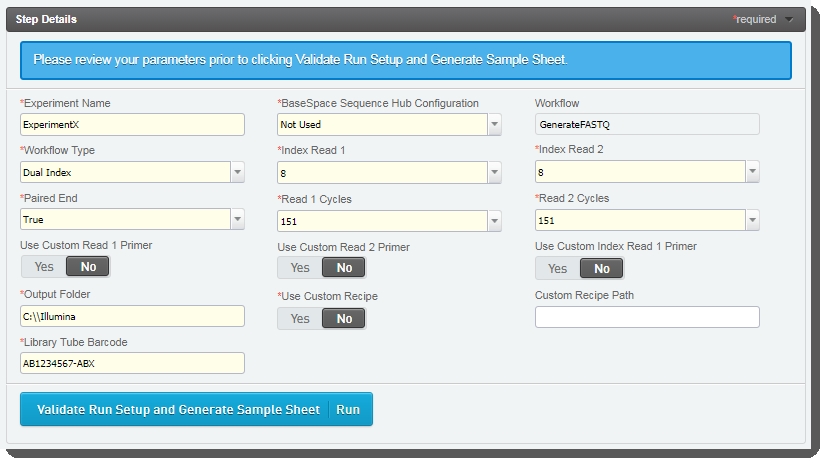
Some of these fields are auto-populated and some must be completed by the user (see the following table for details).
Field
| Value
|
Experiment Name | Enter the experiment name. Only alphanumeric characters, dashes, and underscores are permitted. No spaces. |
BaseSpace Sequence Hub Configuration | Select from preset options: Not Used, Run Monitoring Only, or Run Monitoring and Storage |
Workflow | Automatically populated with preset - GenerateFASTQ. |
Workflow Type | Select from preset options: No Index, Single Index, Dual Index, or Custom |
Index Read 1 | Select from preset options: 0, 6, or 8 - or type a value between 0 and 20. |
Index Read 2 | Select from preset options: 0, 6, or 8 - or type a value between 0 and 20. |
Paired End | Select from preset options: True or False |
Read 1 Cycles | Select from preset options: 251, 151, 101, or 51 - or type a value between 1 and 251. *Value of 251 is only supported for SP flow cell type. For all other flow cell types, maximum value is 151. |
Read 2 Cycles | Select from preset options: 251, 151, 101, or 51 - or type a value between 1 and 251. *Value of 251 is only supported for SP flow cell type. For all other flow cell types, maximum value is 151. |
Use Custom Read 1 Primer | Select if applicable. LIMS v5.0.5 and later: See Configuration Update for NovaSeq Integration with Clarity LIMS v5.0.5 and Later. |
Use Custom Read 2 Primer | Select if applicable. LIMS v5.0.5 and later: See Configuration Update for NovaSeq Integration with Clarity LIMS v5.0.5 and Later. |
Use Custom Index Read 1 Primer | Select if applicable. LIMS v5.0.5 and later: See Configuration Update for NovaSeq Integration with Clarity LIMS v5.0.5 and Later. |
Output Folder | Enter network path for sequencing run folder. For example: \\networkshare\run_data |
Use Custom Recipe | Select if applicable. |
Custom Recipe Path | If you selected the Use Custom Recipe option, enter the path to the custom recipe file to be used. |
Library Tube Barcode | Scan the library tube barcode. |
| 5. | Click Validate Run Setup and Generate Sample Sheet. |
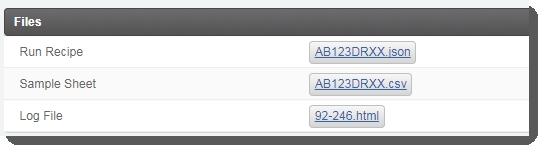
| • | This triggers the automation script, which: Validates the parameters entered on the Record Details screen. |
| • | Generates the sample sheet and attaches it to the placeholder in the Files area of the Record Details screen. |
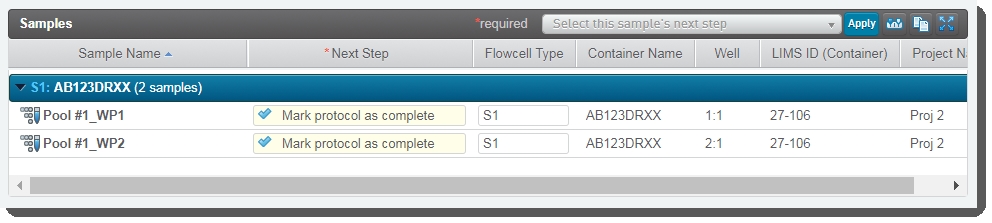
| 6. | On the Assign Next Steps screen, the Next Step field for samples is pre-populated with Mark protocol as complete. |
At this point in the workflow, the user interaction ends. The flow cell is queued for the AUTOMATED - NovaSeq Run (NovaSeq 6000 v3.0) step.
Proceed to Protocol 4: AUTOMATED - NovaSeq Run (NovaSeq 6000 v3.0).
Do not add samples to the Ice Bucket or start the AUTOMATED - NovaSeq Run (NovaSeq 6000 v3.0) step. The integration will do this automatically.
Protocol 4: AUTOMATED - NovaSeq Run (NovaSeq 6000 v3.0)
This protocol contains a single fully-automated step - AUTOMATED - NovaSeq Run (NovaSeq 6000 v3.0).
The integration starts the step automatically and data from the run is parsed back into the LIMS. No user interaction is required. However, the user may open and review the various stages of the step in the LIMS (see Review Run Data
section below).
By default, the Sequencer API is used to set the next step for output samples.
Review Run Data
Read summary metrics are recorded for the library pool. Once the run is complete, you can open the step and review these metrics on the Next Steps screen - in the Step Data section and the Sample Details table.
Step Data Section
You should see values populated in the following step UDFs/master step fields:
| • | Flow Cell Expiration Date |
| • | Instrument Control Software Version |
* Part number of all consumables is not available in the payload sent from the NVCS to the LIMS. This issue will be fixed in the upcoming NVCS release.
Sample Details Table
You should see summary metrics populated in the UDFs/global fields listed below. Values are aggregated across all lanes. Some values - for example Yield PF (Gb) R1 - are summed, while others are averaged.:
| • | Cluster Density (K/mm^2) R1 |
| • | Cluster Density (K/mm^2) R2 |
* For R2 Yield, R2 Error rate, and R2 Q30, there are discrepancies between the values shown in the Illumina SAV software and in the payload sent from the NVCS to the LIMS. This issue will be fixed in the upcoming NVCS release.
How the Integration Works
The following steps summarize how the Sequencer API integration works. For more detailed information, see the NovaSeq 6000 Integration v3.2.1 User and Configuration Guide.
| 1. | When the user begins setting up a run on the NovaSeq instrument, NVCS sends a request for the run recipe. |
| a. | The Sequencer API validates that sample(s) and container(s) are correctly queued for the AUTOMATED - NovaSeq Run (NovaSeq 6000 v3.0) step. |
| b. | The LIMS sends a JSON response to NVCS, which includes both the run recipe information and a link that can be used to download the sample sheet that will later be used with analysis software such as bcl2fastq2. |
| 2. | When the run starts, NVCS sends a RunStarted run status request. The Sequencer API then: |
| • | Validates reagent kit information in the RunStarted request: |
| – | Checks that the reagent kit exists. If it does not, creates it and enables it on the master step. |
| – | Checks that the reagent kit is activated. If it is not, activates it. |
| • | Starts the step for the queued sample(s). The step produces one output file placeholder per lane of the flow cell in use, based on the flow cell type. |
| • | Records all relevant information from the RunStarted request on the step, such as reagent lots and step fields. |
| 3. | At the end of the run, NVCS sends one of the following run status requests: RunCompletedSuccessfully, RunEndedByUSer, or RunErroredOut. The Sequencer API then:Updates the step with any new information. Currently, this includes only the status and cycle/read information. |
| • | Updates the step with any new information. Currently, this includes only the status and cycle/read information. |
| 4. | Once primary analysis completes, NVCS sends a request containing the parsed run metrics. Note that NVCS sends this only when the run completed successfully, but the API does not make this assumption and will accept the request regardless of status. The Sequencer API then: |
| • | Records the metrics into the fields on file placeholder outputs in the LIMS. |
| • | Completes the step in the LIMS if the status is RunCompletedSuccessfully. For all other status options, the step remains in-progress. Note that this is the default autocomplete step behavior. For other configuration options, see the NovaSeq 6000 Integration v3.2.1 User and Configuration Guide. |
Troubleshooting
If an automation trigger does not appear to run its corresponding scripts, see the following articles:
| • | Troubleshooting Automated Informatics in the Clarity LIMS documentation, Automated Informatics section |
| • | Troubleshooting Automation in the API > Clarity LIMS API Overview > API Advanced Topics section |
If an error occurs that does not provide direction on how to proceed, complete the following steps:
| 1. | Confirm the version of the installed Illumina NovaSeq Integration Package by running the following command on the LIMS server command line: |
rpm -qa | grep -i novaseq
| 2. | If the error is related to the AUTOMATED - NovaSeq Run (NovaSeq 6000 v3.0) step, review the log file information. See Logging, below. |
If the automated run step starts, but does not complete:
| 1. | Use one of the following methods to open the in-progress step in the LIMS: |
| • | Log in to the default user account. |
| • | In Lab View, find the step in the Recent Activities sidebar. |
| • | Search for the step in the LIMS using either the Library Tube or Flow Cell barcode as the search term, depending on whether this is a NovaSeq Standard or NovaSeq Xp run. |
| 2. | On the Record Details screen, the Sequencing Log multiline text field will contain logging information. |
| • | If you are unable to reach the Record Details screen, or if the Sequencing Log field does not contain enough information to resolve the issue, review the sequencer-api.log file described below. |
| 3. | Contact the BaseSpace Clarity LIMS support team, supplying the relevant information from the troubleshooting steps already performed. |
Additional troubleshooting information is provided on the Illumina Instrument Integrations FAQ page.
Logging
In addition to updating the Sequencing Log multiline text field on the AUTOMATED - NovaSeq Run (NovaSeq 6000 v3.0) step, the Sequencer API writes a detailed log file to /opt/gls/clarity/tomcat/current/logs/sequencer-api.log.
Log messages include the Library Tube ID and Flow Cell ID whenever the messages are related to a sequencing run-related request. In the LIMS workflows, these IDs are recorded as container names.
Some log messages are not directly related to a sequencing run request, such as downloading the sample sheet. In this case, the file LIMS ID and file name are included in log messages. The sample sheet downloaded by the sequencer will, by default, have a name that includes the sequencing container's ID (Library Tube for Standard or Flow Cell for Xp), so the relevant container name search will find the majority of the log messages for this request as well. However, if you wish to find all messages for downloading a file, then you must know the LIMS ID of the file. You can find this by looking in the LIMS API and, for sample sheets used in a run, in the recipe response (which should also be found in the log file).
Below are some example lines from the log file:
2018-06-19 21:32:19.705 INFO --- [] SequencerAPIApplication : Started SequencerAPIApplication in 7.026 seconds (JVM running for 15.964)
...
2018-06-19 21:34:44.564 INFO --- [Library Tube Id=NV1234567-LIB, Flow Cell Id=RM123DSXX] LimsApiLookupUtil : Searching for containers with name 'NV1234567-LIB' or 'RM123DSXX'
....
2018-06-19 21:35:27.158 INFO --- [] FileService : Successfully retrieved file 'NV1234567-LIB.csv' with LIMS ID 40-51 through the API.


























![]()














![]()











