Testing Volume Accuracy of the IAPS with IAC Tips
This protocol uses fluorescein dye to measure quantities of liquid delivered by the IAPS with IAC compared to a manually derived standard curve. It is critical that the fluorescein dyes used for the standard curve and the IAPS with IAC quantification are derived from the same starting dilution of stock. Failure can lead to erroneous assay results.
This section explains how to prepare reagents for testing the IAPS with IAC.
Fluorescein Stock
| 1. | Weigh out 25 mg fluorescein into a 100 ml bottle. |
| 2. | Slowly add 25 ml DMSO. |
| 3. | Mix thoroughly. |
| 4. | Store at room temperature protected from light. |
Fluorescein Dilution Buffer
| 1. | Prepare 1X TE (Tris s acid—EDTA) |
| • | Add 10 ml 100X TE (1M Tris-HCl, 0.1M EDTA) to 990 ml DI H2O in a 1 L bottle. |
| • | Mix thoroughly. |
| • | Store at room temperature. |
| 2. | Prepare 10% Tween 80 |
| • | Weigh 107.4 grams Tween 80. |
Tween 80 is extremely viscous. Weight measurement is more accurate than liquid volume measurement.
| • | Add the Tween 80 to the 1 L bottle. |
| • | Dissolve in DI H2O to 1000 ml. |
| • | Mix thoroughly. |
| • | Store at room temperature. |
| 3. | Prepare 1X TE with 0.01% Tween 80 |
| • | Add 1 ml 10% Tween 80 to 1000 ml 1X TE buffer. |
| • | Mix thoroughly. |
| • | Store at room temperature. |
High-Concentration Fluorescein
| 1. | In a 100 ml bottle, add 25 ml fluorescein stock in DMSO (1.0 mg/ml) to 75 ml fluorescein dilution buffer (1X TE with 0.01% Tween 80). |
| 2. | Mix thoroughly. |
| 3. | Discard after use. |
Low-Concentration Fluorescein
| 1. | In a 500 ml bottle, add 50 ml newly prepared 0.25 mg/ml high-concentration fluorescein to 450 ml fluorescein dilution buffer (1X TE with 0.01% Tween 80). |
| 2. | Mix thoroughly. |
| 3. | Discard after use. |
| 1. | Label a new FLUOTRAC 200 plate Ind-Col Dispense. |
| 2. | Label another new FLUOTRAC 200 plate Multi-Col Dispense. |
| 3. | From the IAPS with IAC control computer, open the Illumina Automation Control Software. |
| 4. | Select Robot QC Tasks | 8-Tip Robot QC. |
| 5. | Add 35 ml fluorescein dilution buffer to a quarter reservoir. Place the reservoir in position A of the reservoir frame, as shown on the IAPS with IAC worktable map. |
| 6. | Add 6 ml low-concentration fluorescein (0.025 mg/ml) to a quarter module reservoir and place the reservoir in position B. |
| 7. | Place the FLUOTRAC 200 plates on the IAPS with IAC worktable according to the IAPS with IAC worktable map. |
| 8. | Make sure that: |
| • | All items are placed properly on the IAPS with IAC worktable. |
| • | All caps and seals have been removed. |
| • | For each plate, well A1 is in the upper left corner of the frame. |
| 9. | Select Run. |
The IAPS with IAC conducts an internal QC process. A message in the lower status bar indicates when the QC process is complete.
| 10. | Remove the FLUOTRAC 200 plates from the IAPS with IAC worktable and cover with aluminum foil. |
| 11. | Dispose of any remaining reagents in accordance with your facility requirements. |
| 12. | Select Robot QC Tasks | Robot Bleach Wash. |
| 13. | Add 5 ml 10% bleach to a quarter reservoir. |
| 14. | Place the reservoir in position A of the reservoir frame, as shown on the IAPS with IAC worktable map. |
| 15. | Select Run. |
| 16. | When the tip bleach process is complete, select Sys Flush. |
| 17. | Observe the lines for air bubbles. |
| 18. | Repeat the system flush process at least three times, until the lines are free of air bubbles. |
If bubbles persist in the lines, contact Illumina Technical Support.
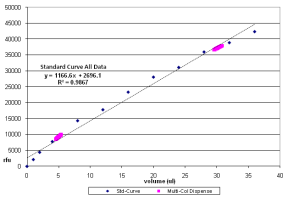
The Standard plate is used to generate a standard curve of fluorescent units versus volume dispensed. You create the Standard plate by manually pipetting fluorescein over a range of volumes (1–36 µl) into 100 µl fluorescein dilution buffer. The Standard plate is then used to calculate volumes dispensed by the IAPS with IAC into the IAPS with IAC QC test plates.
To ensure successful generation of the standard curve, use calibrated pipettes.
| 1. | Label a FLUOTRAC 200 plate Standard Plate. |
| 2. | Dispense 12 ml fluorescein dilution buffer into a disposable multichannel trough. |
| 3. | Using a multichannel pipette, add 100 µl fluorescein dilution buffer into each well of the Standard plate. |
| 4. | Add 2 ml low-fluorescein concentration standard (0.025 mg/ml) to a disposable multichannel reservoir. |
| 5. | Using an 8-channel pipette, add low-fluorescein concentration standard (0.025 mg/ml) into each well of the Standard plate according to the volumes shown in . |
| 6. | Cover the plate and store it in the dark until reading on the fluorometer. |
| 1. | Read the fluorescence intensities of the Standard and the IAPS with IAC QC plates on a fluorometer at an excitation of 485 nm and 538 nm emission. |
| 2. | Set the fluorometer to Automix for 10 seconds before the first reading. |
| 1. | Open the QC Analysis Tool Excel workbook. |
| 2. | Select the Standard Data Input sheet. Enter the fluorescein intensities of the standard curve, following the template in the Excel workbook. |
The standard curve statistics generate automatically.
| 3. | Make sure that the standard curve R2 for the low and the high dispense volumes are ≥ 0.99. |
If the values are < 0.99, the standard curve must be repeated. If the standard curve is < 0.99, the cell for that curve is highlighted as failing.
| 4. | Select the Individual Tips Data Input sheet. Enter the fluorescein intensities for the Ind-Col Dispense Robot QC plate, following the template in the Excel workbook. |
| 5. | Select the Multi Tips Data Input sheet. Enter the fluorescein intensities for the Multi-Col Dispense Robot QC plate, following the template in the Excel workbook. |
| 6. | Select the Summary Analysis sheet and review the pass/fail data. |
| 7. | Tips that do not meet the required QC specifications are flagged with red. If a tip is failing, perform a Bleach Wash followed by a System Flush on the IAPS with IAC, and then repeat the Robot QC procedure. |
| 8. | Evaluate the individual and multicolumn dispense results on the standard curve. |
The acceptable standard curve R2 for the low and the high dispense volumes is ≥ 0.99.
Standard Curve with Individual Column Dispense
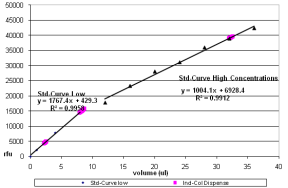
Standard Curve with Multi-Tip Column Dispense