Extend and Stain BeadChips
This step washes unhybridized and nonspecifically hybridized DNA samples from the BeadChips, adds labeled nucleotides to extend primers hybridized to the sample, and stains the primers. After the flow-through chambers are disassembled, the BeadChips are coated for protection.
| • | 70% EtOH (Ethanol) |
| • | 95% formamide/1 mM EDTA ( |
| • | ATM (1 tube/4 BeadChips) |
| • | TEM (1 tube/4 BeadChips) |
| • | PB1 (310 ml for 1–8 BeadChips) |
| • | RA1 (10 ml for 1–8 BeadChips) |
| • | STM (1 tube/4 BeadChips) |
| • | XC1 (1 tube/4 BeadChips) |
| • | XC2 (1 tube/4 BeadChips) |
| • | XC3 ( |
| • | Resuspended XC4 (310 ml for 1–8 BeadChips, 285 ml for 9–24 BeadChips) |
| • | Make sure that the label of each STM tube indicates the same stain temperature. |
| • | Decant only the necessary volume of reagent. |
| • | Use fresh RA1 for each step that requires it. Properly stored RA1 that has not been dispensed for the previous resuspension step or this extend and stain step is considered fresh. |
| • | RA1 might form visible precipitate or crystals. Before each use, hold in front of a light and inspect. Invert several times to redissolve the solution as needed. |
| • | The XC4 coat is slippery and makes the BeadChips difficult to hold. Self-locking tweezers grip the BeadChip firmly and help prevent damage. |
This set of reagents contains potentially hazardous chemicals. Personal injury can occur through inhalation, ingestion, skin contact, and eye contact. Ventilation should be appropriate for handling of hazardous materials in reagents. Wear protective equipment, including eye protection, gloves, and laboratory coat appropriate for risk of exposure. Handle used reagents as chemical waste and discard in accordance with applicable regional, national, and local laws and regulations. For additional environmental, health, and safety information, refer to the SDS at support.illumina.com/sds.html.
| 1. | Prepare the following consumable. |
|
Item |
Storage |
Instructions |
|---|---|---|
|
RA1 |
-25°C to -15°C |
Thaw at room temperature. |
| 2. | Place reagent tubes in a rack in the order of use. |
Placement of Reagent Tubes in Rack
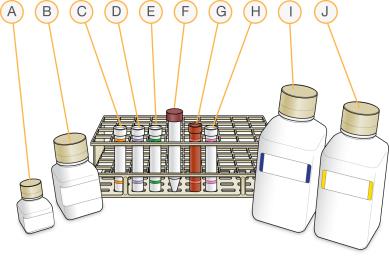
| A. | RA1 |
| B. | XC3 |
| C. | XC1 |
| D. | XC2 |
| E. | TEM |
| F. | 95% Formamide / 1 mM EDTA |
| G. | STM |
| H. | ATM |
| I. | PB1 |
| J. | XC4 |
Procedure
| 1. | Make sure that the water circulator is filled to the appropriate level. Refer to the manufacturer instructions. |
| 2. | At the robot PC, select Robot QC Tasks, and then select Circulator Manager to set the water circulator to 44°C: |
| a. | In the Action section drop-down list, select Set Target Temperature. |
| b. | In the Set Target Temperature field, enter 44. |
| c. | Select Execute. |
Chamber Rack Setup
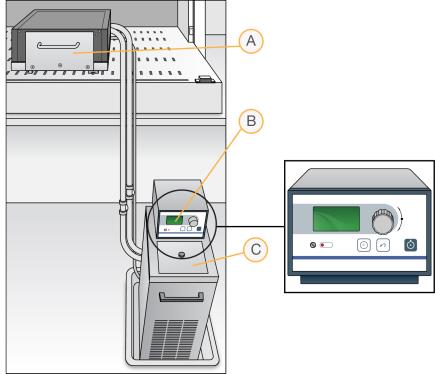
| A. | Chamber rack |
| B. | Water circulator with programmable temperature controls |
| C. | Reservoir cover |
| 3. | Confirm the temperature using the chamber rack temperature probe. |
The temperature displayed on the water circulator screen can differ from the chamber rack temperature.
| 4. | Remove bubbles trapped in the chamber rack. |
| a. | Separate the heat exchanger from the reagent pan. |
| b. | Lift the heat exchanger upright and away from you with the tubing at the bottom, and turn 90° counter clockwise. |
| c. | Return the heat exchanger to a horizontal position. |
| d. | Repeat steps b and c 3 times for a total of 4 rotations or until all bubbles are removed. |
| e. | Using Kimwipes dampened with laboratory-grade water, clean all surfaces between the heat exchanger and reagent pan. Discard Kimwipes with formamide waste. |
| f. | Place the Te-Flow back on the reagent pan. Using the two guide pins in the reagent pan, make sure that the Te-Flow is flush. |
| 5. | Using the Illumina temperature probe, test at least three locations on the chamber rack. |
| a. | For accurate measurements, make sure that the temperature probe touches the base of the chamber rack. |
| b. | Make sure that all locations are at 44°C ±0.5°C. |
| c. | If the temperature is not within ±0.5°C, adjust the water circulator control knob to reach 44°C ±0.5°C. |
| d. | Do not leave the temperature probe in the first three rows of the chamber rack. Reserve these rows for BeadChips. |
Temperature Probe in Chamber Rack

The following steps must be performed without
| 1. | At the robot PC, select XStain Tasks | XStain HD BeadChip. |
| 2. | If you are imaging the BeadChip immediately after the staining process, turn on the scanner to allow the lasers to stabilize. |
| 3. | Place reservoirs on the robot worktable according to the worktable map, and add reagents to reservoirs as follows. |
|
Reagent |
# BeadChips |
Volume |
|---|---|---|
|
95% formamide/1 mM EDTA |
1–8 |
15 ml |
|
9–16 |
17 ml |
|
|
17–24 |
25 ml |
|
|
RA1 |
1–8 |
10 ml |
|
9–16 |
20 ml |
|
|
17–24 |
30 ml |
|
|
XC3 |
1–8 |
50 ml |
|
9–16 |
100 ml |
|
|
17–24 |
150 ml |
| 4. | Invert the XC1, XC2, TEM, STM, and ATM tubes to mix. Remove the caps, and place on the robot worktable according to the worktable map. |
Robot Setup for Single-Base Extension and Stain
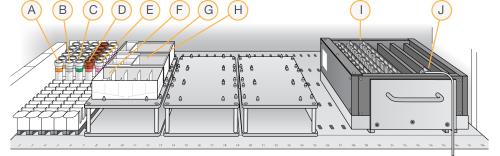
| A. | XC1 Tubes |
| B. | XC2 Tubes |
| C. | TEM Tubes |
| D. | STM Tubes |
| E. | ATM Tubes |
| F. | XC3 in Full Reservoir |
| G. | RA1 in Half Reservoir |
| H. | 95% Formamide/1 mM EDTA in Quarter Reservoir |
| I. | 24 BeadChips in Chamber Rack |
| J. | Temperature Probe |
| 5. | In the Basic Run Parameters pane, enter the number of BeadChips, up to 24. |
| 6. | Select Run. |
| 7. | When prompted, enter the stain temperature listed on the STM tube. Do not load the BeadChips yet. |
| 8. | When the chamber rack reaches 44°C, place the flow-through chambers into the chamber rack, according to the robot worktable map. |
Start the robot immediately to prevent BeadChips from drying.
| 9. | At the robot PC, select OK. |
| 10. | When the robot finishes, remove the flow-through chambers from the chamber rack, and place them horizontally on the lab bench at room temperature. |
| 1. | Gather the following equipment: |
| • | Kimwipes, large |
| • | Staining rack |
| • | Self-locking tweezers |
| • | Tube rack |
| • | Vacuum desiccator |
| • | Vacuum hose |
| • | Wash dishes (2) |
| 2. | During the procedure, prevent dust or lint from entering the wash dishes. |
| • | Clean wash dishes with low-pressure air before use. |
| • | Cover wash dishes with wash dish covers when not in use. |
| 3. | Wash the tube racks and wash dishes thoroughly after each use. |
| • | Rinse with deionized water. |
| • | Dry racks and wash dishes upside down on a wash rack. |
| 4. | Place a clean tube rack on top of several layers of Kimwipes or an absorbent pad. |
After the staining rack containing BeadChips is removed from the XC4 wash dish, it is placed on this rack.
| 5. | Prepare another clean tube rack that fits the internal dimensions of vacuum desiccator for removal of the BeadChips. Allow one rack per eight BeadChips. |
Kimwipes are not needed under this tube rack.
| 6. | Set up two top-loading wash dishes labeled PB1 and XC4. |
| 7. | Indicate the fill volume of each wash dish as follows. |
| a. | Add 310 ml water. |
| b. | Mark the water level on the side. |
| c. | Empty the water. |
Indicating fill volume before adding reagents allows reagents to be added directly from the bottles, minimizing contamination.
Wash Dish Setup
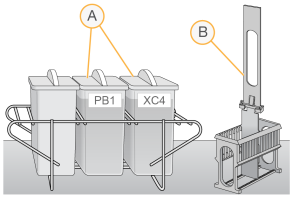
| A. | Labeled and filled wash dishes |
| B. | Staining rack |
| 8. | Add 310 ml PB1 to the PB1 wash dish. |
| 9. | Submerge the staining rack in the wash dish so that the locking arms and tab face you. |
This orientation allows you to remove the BeadChips safely.
Staining Rack Components
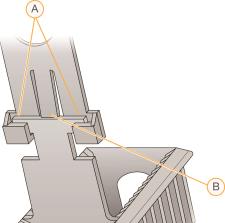
| A. | Locking arms |
| B. | Tab |
| 10. | Leave the staining rack in the wash dish for later use (carrying the BeadChips after disassembling the flow-through chambers). |
| 11. | Using the dismantling tool, remove the two metal clamps from a flow-through chamber. |
The dismantling tool prevents chipping the glass back plates.
Using Dismantling Tool to Remove Metal Clamps
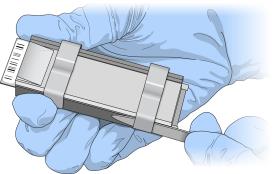
| 12. | Lift the glass back plate straight up to remove. Set aside for cleaning after finishing this procedure. |
Sliding the glass along the BeadChip can damage the BeadChip.
| 13. | Remove the spacer, avoiding contact with the BeadChip stripes. |
| 14. | Remove the BeadChip from the black frame. Handle the BeadChip only by the barcode end or edges. |
| 15. | Repeat steps 11–14 to disassemble each flow-through chamber one at a time. |
| 16. | Place the BeadChips into the submerged staining rack. Make sure that the BeadChip barcodes face away from you and the locking arms face toward you. |
Submerge each BeadChip as quickly as possible to prevent drying.
| 17. | If necessary to seat a BeadChip, briefly lift the staining rack from the wash dish and seat the BeadChip. |
| 18. | Make sure that the BeadChips are submerged. |
| 19. | Slowly move the staining rack up and down 10 times, breaking the PB1 surface. If the tops of the BeadChips touch, gently wiggle the staining rack to separate the slides. |
Free circulation of PB1 between BeadChips is important.
Submerging of BeadChips with Staining Rack
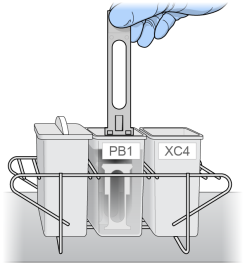
| 20. | Soak for 5 minutes. |
| 21. | Vigorously shake the XC4 bottle to resuspend completely. If necessary, vortex until dissolved. |
| 22. | Add 310 ml XC4 to the XC4 wash dish. |
| • | Cover to prevent lint or dust from entering. |
| • | Do not let sit for more than 10 minutes. |
| 23. | Transfer the staining rack from the PB1 wash dish to the XC4 wash dish. |
Transfer of Staining Rack to XC4 Wash Dish
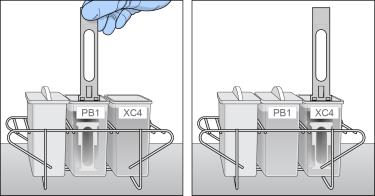
| 24. | Slowly lift the staining rack up and down 10 times, breaking the XC4 surface. If the tops of the BeadChips touch, gently wiggle the staining rack to separate the slides. |
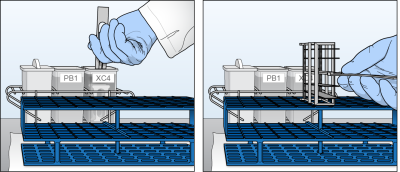
| 25. | Soak for 5 minutes. |
| 26. | Remove the staining rack in one quick motion and place it onto the prepared tube rack. |
Removal of Staining Rack
| 27. | Make sure that the staining rack is in the center of the tube rack to ensure uniform coating. Avoid the raised edges. |
Staining Rack in Tube Rack Center
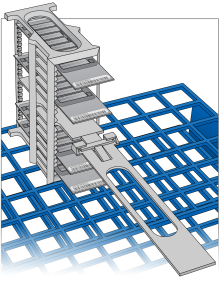
| 28. | [Optional] To facilitate BeadChip removal, remove the staining rack handle as follows. |
| a. | Holding the top of the staining rack in position, grasp the handle between your thumb and forefinger. |
| b. | Push up the tab with your thumb and push the handle away from you, unlocking the handle. |
| c. | Pull up the handle and remove. |
| 29. | Working from top to bottom, place each BeadChip as follows. |
| a. | Holding the staining rack handle (if present), use self-locking tweezers to grip the BeadChip by the barcode end. |
| b. | Place the BeadChip onto a tube rack with the barcode facing up and toward you. Do not place on the bottom rack or allow BeadChips to rest on the tube rack edge or touch each other. |
Placement of BeadChips on Tube Rack
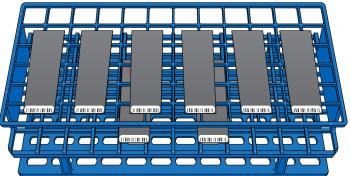
Proper BeadChip placement prevents wicking, uneven drying, and pooled dye protectant.
| 30. | Place the tube rack into the vacuum desiccator. |
Each desiccator can hold one tube rack (eight BeadChips).
| 31. | Make sure that the vacuum desiccator valve is seated tightly and securely, and remove the red plug from the three-way valve. |
| 32. | Gently lift the vacuum desiccator lid to ensure proper sealing. Make sure that the lid does not lift off the desiccator base. |
| 33. | Dry the BeadChips in the vacuum desiccator for 50–55 minutes at 675 mm Hg (0.9 bar). |
Drying times can vary according to room temperature and humidity.
| 34. | Release the vacuum by turning the handle slowly. |
Make sure that air enters the desiccator slowly to avoid disturbing the contents. Improper use of the vacuum desiccator can result in damage to the BeadChips, especially if you remove the valve plug while a vacuum is applied. For detailed vacuum desiccator instructions, refer to the documentation included with the desiccator.
| 35. | Return the desiccator to storage. Store with the red valve plug in the three-way valve of the desiccator to prevent dust and lint from accumulating in the valve port. |
| 36. | Touch the edges of the BeadChips (do not touch arrays) to make sure etched, barcoded sides are dry. |
| 37. | Clean the back of each BeadChip using a Kimwipe sprayed with 70% EtOH. |
| a. | Hold the BeadChip at a downward angle to prevent excess EtOH from dripping onto the stripes. |
| b. | Without touching the stripes, wipe the underside of the BeadChip until XC4 is removed (5–6 times). |
| 38. | Clean the glass back plates. |
For instructions, refer to the Infinium Lab Setup and Best Practices (document # 11322460).
SAFE STOPPING POINT
Store the BeadChips in the Illumina BeadChip Slide Storage Box at room temperature in the dark. Scan within 72 hours.
