Convert DNA
In this step, bisulfite converts genomic DNA samples using the Zymo EZ-96 DNA Methylation-Lightning MagPrep Kit. The bisulfite-converted genomic DNA (BCD) samples are then transferred to the BCD plate.
Make sure to follow the manufacturer instructions.
A minimum of 250 ng fresh DNA is supported for this reaction. However, using more DNA, from 500 ng to 1000 ng, results in higher reproducibility.
 Consumables
Consumables
|
•
|
Zymo EZ-96 DNA Methylation-Lightning MagPrep Kit
|
|
–
|
Manual catalog # D5046 (4x96 rxns) or D5047 (8x96 rxns) |
|
–
|
Automated catalog # D5049 (1x96 rxns) |
|
•
|
96-well 0.2 ml skirted microplate (1–3 plates) |
 About Reagent
About Reagent
|
•
|
Use a Zymo EZ DNA Methylation-Lightning MagPrep Kit (catalog # D5046, D5047, or D5049) for bisulfite conversion of genomic DNA. Bisulfite conversion kits not specified in this guide might not be supported for use with the Infinium HD Methylation Assay. For specifics, contact Illumina Technical Support. |
|
•
|
The conversion reagent is photosensitive. Make sure to minimize exposure to light. |
 Preparation
Preparation
|
1.
|
Each bisulfite conversion process requires the following: |
|
2.
|
Prepare the conversion reagent according to the manufacturer instructions for immediate use. |
|
3.
|
Prepare the wash buffer according to the manufacturer instructions. |
|
4.
|
Apply a BCD barcode label to each new 96-well 0.2 ml skirted microplate. |
Procedure
Use the instructions in the Zymo EZ DNA Methylation-Lightning Kit to do the following steps:
 Add Bisulfite-Conversion Reagent and Incubate
Add Bisulfite-Conversion Reagent and Incubate
|
1.
|
Add 130 µl Lightning Conversion Reagent to 20 µl DNA sample in a conversion plate. |
|
2.
|
Incubate in a thermal cycler using the following settings: |
|
3.
|
[Optional] Hold DNA at 4°C for up to 20 hours in the thermal cycler. |
 Clean Up Conversion Reagent
Clean Up Conversion Reagent
|
1.
|
Prepare the collection plate with the provided 600 µl M-binding buffer and 10 µl MagBinding beads. |
|
2.
|
Transfer and mix samples from the conversion plate to the collection plate. |
|
3.
|
Incubate the plate at room temperature for 5 minutes. |
|
4.
|
Transfer the plate to the magnetic stand. Wait 5 minutes. |
|
5.
|
Remove the supernatant to clean up the conversion reagent. |
|
6.
|
Remove the plate from the magnetic stand. |
|
7.
|
Add 400 µl M-Wash buffer and resuspend the beads. |
|
8.
|
Transfer the plate to the magnetic stand. Wait 3 minutes. |
|
9.
|
Remove the supernatant to clean up the M-Wash buffer. |
 Desulphonate Samples
Desulphonate Samples
|
1.
|
Desulphonate the samples with 200 µl L-desulphonation buffer. |
|
2.
|
Incubate at room temperature for 15–20 minutes. |
|
3.
|
Transfer the plate to the magnetic stand. Wait 3 minutes. |
|
4.
|
Remove the supernatant to clean up the L-desulphonation buffer. |
 Wash Plate
Wash Plate
|
1.
|
Add 400 µl M-Wash buffer and resuspend the beads. |
|
2.
|
Transfer the plate to the magnetic stand. Wait 3 minutes. |
|
3.
|
Remove the supernatant to clean up the M-Wash buffer. |
|
4.
|
Repeat steps 1–3 one time. |
|
5.
|
To dry the beads, transfer the plate to a heating element at 55°C for 20–30 minutes and remove residual M-wash buffer. |
 Elute Bisulfite-Converted DNA
Elute Bisulfite-Converted DNA
|
1.
|
Add 25 µl elution buffer directly to the dried beads and resuspend. |
|
2.
|
Heat the elution at 55°C for 4 minutes. |
|
3.
|
Transfer the plate to a magnetic stand for 1 minute or until the beads pellet. |
|
4.
|
Collect the supernatant and transfer it to a clean elution plate. |
 Create the BCD Plate
Create the BCD Plate
|
1.
|
If frozen, thaw BCD samples to room temperature and vortex to mix. |
|
2.
|
Apply a BCD barcode label to a new 0.8 ml MIDI plate or a new 0.2 ml TCY plate. |
|
3.
|
Transfer the BCD to the plate. Use the volume appropriate for your plate: |
|
•
|
MIDI plate: 20 µl BCD sample to each well |
|
•
|
TCY plate: 10 µl BCD sample to each well |
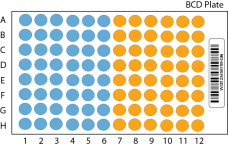
The example illustrates 96 samples. For 48 samples, fill only the blue side of the plate.
BCD Plate Sample Well Distribution
SAFE STOPPING POINT
If you are stopping, heat-seal the plate, and store at -25°C to -15°C for up to 30 days.