Hybridize DNA to the BeadChip
This step dispenses the fragmented, resuspended DNA onto BeadChips. Incubation then hybridizes each DNA sample to a section of the BeadChip.
Before hybridizing DNA to the BeadChip, make sure that the DMAP files have been downloaded for the BeadChips that are used.
| • | PB2 |
| • | XC4 |
| • | 1% aqueous Alconox solution |
| • | DI water |
| • | 100% EtOH |
| 1. | If the MSA4 plate was frozen, prepare it as follows. |
| a. | Thaw at room temperature. |
| b. | Centrifuge at 280 × g for 1 minute. |
| 2. | Remove BeadChips from storage, but do not unpackage. |
| 3. | Make sure that the DMAP files are downloaded for each BeadChip that is used. |
| 4. | Preheat the heat block to 95°C. |
| 5. | Preheat the Illumina Hybridization Oven to 48°C. |
Procedure
| 1. | Place the MSA4 plate at 95°C on the preheated heat block for 20 minutes to denature the DNA. |
| 2. | Cool the MSA4 plate on the benchtop at room temperature for 30 minutes. |
| 3. | Pulse the centrifuge at 280 × g. |
| 1. | Place the EX/XT hybridization chamber lids, hybridization chamber gaskets, and hybridization chamber bases on the benchtop. |
Hybridization Chamber Components
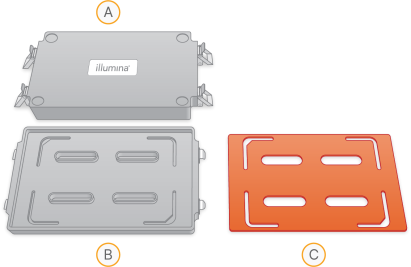
| A. | Hybridization chamber lid |
| B. | Hybridization chamber base |
| C. | Hybridization chamber gasket |
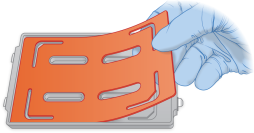
| 2. | Align the wide cutout of the gasket to the wide cutout on the hybridization chamber. |
| 3. | Press the gasket down around the edges to make sure that the gasket is properly seated into the hybridization chamber base. |
Placing of Gaskets on Hybridization Chamber Base
| 4. | Dispense 800 µl PB2 into each of the 4 humidifying buffer reservoirs of the EX/XT hybridization chamber bases. |
PB2 Addition
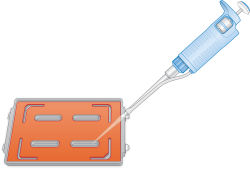
| 5. | Immediately cover the chamber base with the lid to prevent evaporation. |
Locking the lid is not necessary.
| 6. | Leave the EX/XT hybridization chambers covered and on the benchtop at room temperature until the BeadChips are loaded with DNA (~1 hour). |
| 1. | Ensure BeadChips have reached room temperature for 30 minutes. Remove the BeadChips from all packaging by holding BeadChips by the ends away from the sample inlets. |
| 2. | Place BeadChips into the Dual Hybridization Insert and Baseplate so that the barcode ends align. |
BeadChip Placement
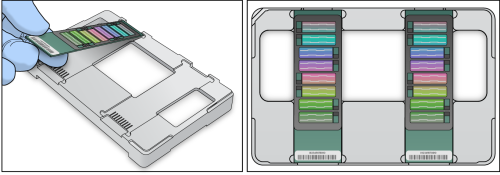
BeadChips must be transferred to hybridization chambers immediately at the end of the next procedure. Do not begin the Execute Run step if you cannot immediately transfer the BeadChips.
| 3. | Open IAPS with ILASS. |
| 4. | Select Infinium HD Methylation Assay, and then Hybridize DNA to the BeadChip. |
| 5. | Select the Product Type drop-down, and then select Infinium Methylation Array HD-8. |
| 6. | In the Container Input Quantity box, enter the number of 96-well plates to be processed. You can manually enter the value or set the value using +/-. |
ILASS displays the number of required materials based on number of plates.
| 7. | Select Continue. |
| 8. | Set up the IAPS with ILASS worktable as shown on the interface, according to number of plates and BeadChips/BeadChip type used in the run. |
Worktable Setup
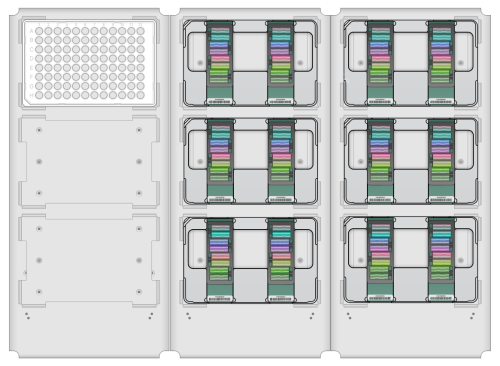
Transfer BeadChips to hybridization chambers immediately after starting the robot. Do not let the BeadChips dry out. Do not start the robot if you cannot immediately transfer the BeadChips.
| 9. | Remove the foil seal from the MSA4 plate and load the plate onto the IAPS with ILASS worktable. |
| 10. | Load BeadChips on baseplates onto the IAPS with ILASS worktable according to the interface. |
| 1. | Select Auto-Scan Item(s). |
If necessary, after scanning, follow the ILASS user interface to rescan (
| 2. | After successful scanning, ILASS displays a timer. |
| 3. | Select Execute Run. |
BeadChips must be removed from the robot worktable immediately to prevent excess sample evaporation. Proceed to the next section Hybridize DNA to the BeadChip immediately.
| 4. | Wait for the Infinium Automated Pipetting System with Illumina Lab Automation Software Solution to finish the run. |
| 5. | Seal the MSA4 plates with heat seal or foil adhesive and freeze at -20°C. |
| 1. | Make sure that the Illumina Hybridization Oven is set to 48°C. |
Keep hybridization chambers at room temperature when you load the BeadChips. Do not place the hybridization chamber in the Illumina Hybridization Oven when loading the BeadChips.
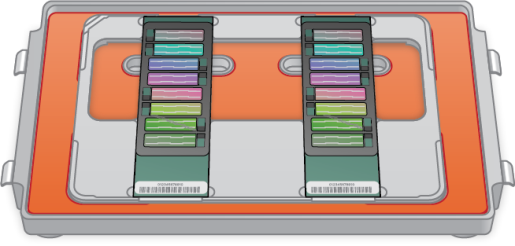
| 2. | Load the BeadChip baseplates into the hybridization chamber. Up to three BeadChip baseplates can be stacked per hybridization chamber. |
| • | Position the barcode end over the ridges indicated on the chamber. |
| • | Keep the baseplates steady and level. |
Loading Baseplates into Chamber
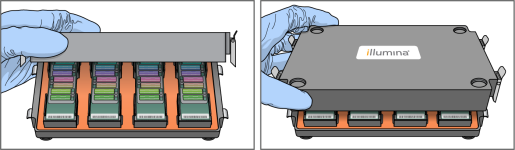
| 3. | Place the back of the lid onto the chamber, and then slowly lower the front to avoid dislodging the baseplates. |
Lowering of Chamber Lid
| 4. | Close all four clamps so that the lid is secure and sits evenly on the base without any gaps. |
Close the clamps in the following order: top-left, bottom-right, top-right, bottom-left.
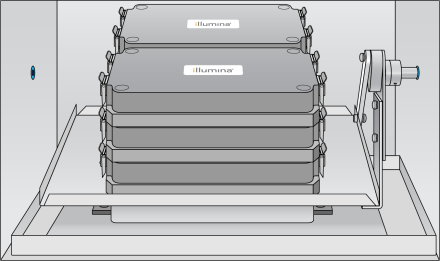
| 5. | Place the chamber into the preheated Illumina Hybridization Oven so that the top logo faces you. |
You can stack up to three chambers per row for a total of six chambers. Make sure that the feet of the top chamber fit into the indents on the bottom chamber.
Chamber in Illumina Hybridization Oven
| 6. | Incubate overnight at 48°C for 16–24 hours. |
| 1. | Add 330 ml fresh 100% EtOH to the XC4 bottle. |
The resulting volume is ~350 ml. Each XC4 bottle can process up to 48 BeadChips.
| 2. | On the XC4 bottle label, record that the EtOH has been added. |
| 3. | Vigorously shake to resuspend. If needed, vortex at 1625 rpm to complete suspension. |
| 4. | Leave the bottle upright on the lab bench overnight. |
If XC4 was not left to resuspend overnight, you can still proceed with the assay.
