Convert DNA
This step bisulfite converts genomic DNA samples using the Zymo EZ-96 DNA Methylation Lightning Kit. The bisulfite-converted genomic DNA (BCD) samples are then transferred to the BCD plate.
Make sure to follow the manufacturer instructions.
A minimum of 250 ng fresh DNA is supported for this reaction. Using more DNA, from 500 ng to 1000 ng, results in higher reproducibility.
| • | Zymo EZ-96 DNA Methylation Lightning Kit (Manual catalog # D5046 (4x96 rxns) or D5047 (8x96 rxns), |
| • | 96-well 0.2 ml skirted microplate (1–3 plates) |
| • | Genomic DNA (for HTS Methylation) |
The following diagram illustrates the bisulfite conversion workflow.
Each bisulfite conversion process requires the following:
| • | gDNA (> 250 ng) |
| • |
|
| 1. | Prepare the conversion reagent according to the manufacturer instructions for immediate use. |
| 2. | Prepare the wash buffer according to the manufacturer instructions. |
| 3. | Apply a BCD barcode label to each new 96-well 0.2 ml skirted microplate. |
Use the instructions in the Zymo EZ DNA Methylation-Lightning Kit to do the following steps:
| 1. | Add 130 µl Lightning Conversion Reagent to 20 µl DNA sample in a conversion plate. |
| 2. | Incubate in a thermal cycler using the following settings: |
| • | 98°C for 8 minutes |
| • | 54°C for 1 hour |
| 3. | [Optional] Hold DNA at 4°C for up to 20 hours in the thermal cycler. |
| 1. | Prepare the collection plate with the provided 600 µl M-binding buffer and 10 µl MagBinding beads. |
| 2. | Transfer and mix samples from the conversion plate to the collection plate. |
| 3. | Incubate the plate at room temperature for 5 minutes. |
| 4. | Transfer the plate to the magnetic stand. Wait 5 minutes. |
| 5. | Remove the supernatant to clean up the conversion reagent. |
| 6. | Remove the plate from the magnetic stand. |
| 7. | Add 400 µl M-Wash buffer and resuspend the beads. |
| 8. | Transfer the plate to the magnetic stand. Wait 3 minutes. |
| 9. | Remove the supernatant to clean up the M-Wash buffer. |
| 1. | Desulphonate the samples with 200 µl L-desulphonation buffer. |
| 2. | Incubate at room temperature for 15–20 minutes. |
| 3. | Transfer the plate to the magnetic stand. Wait 3 minutes. |
| 4. | Remove the supernatant to clean up the L-desulphonation buffer. |
| 1. | Add 400 µl M-Wash buffer and resuspend the beads. |
| 2. | Transfer the plate to the magnetic stand. Wait 3 minutes. |
| 3. | Remove the supernatant to clean up the M-Wash buffer. |
| 4. | Repeat steps 1–3 one time. |
| 5. | To dry the beads, transfer the plate to a heating element at 55°C for 20–30 minutes and remove residual M-wash buffer. |
| 1. | Add 25 µl elution buffer directly to the dried beads and resuspend. |
| 2. | Heat the elution at 55°C for 4 minutes. |
| 3. | Transfer the plate to a magnetic stand for 1 minute or until the beads pellet. |
| 4. | Collect the supernatant and transfer it to a clean elution plate. |
| 1. |
|
| 2. | Apply a BCD barcode label to a new 0.8 ml MIDI plate or a new 0.2 ml TCY plate. |
| 3. | Transfer the BCD to the plate as follows. |
| • | MIDI plate: 20 µl BCD sample to each well |
| • | TCY plate: 10 µl BCD sample to each well |
The example illustrates 96 samples. For 48 samples, fill only the blue side of the plate.
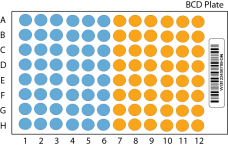
SAFE STOPPING POINT
If you are stopping, heat-seal the plate, and store at -25°C to -15°C for up to 30 days.
