Initiating a Standard SBS Sequencing Run
This step initiates a standard SBS sequencing run in one of four modes:
| • | Cloud mode—Run is selected from a list of planned runs in NextSeq 1000/2000 Control Software. During sequencing, CBCL data is uploaded to BaseSpace Sequence Hub. After sequencing, DRAGEN in BaseSpace Sequence Hub starts automatically. |
| • | Hybrid mode—Run is selected from a list of planned runs in NextSeq 1000/2000 Control Software. After sequencing, on-instrument analysis starts automatically. CBCL data and DRAGEN secondary analysis output files are stored in the selected output folder. |
| • | Local mode—A sample sheet in v2 file format is manually imported into NextSeq 1000/2000 Control Software. After sequencing, on-instrument analysis starts automatically. CBCL data and DRAGEN secondary analysis output files are stored in the selected output folder. If Proactive, Run Monitoring and Storage is selected, analysis can also be initiated through BaseSpace Sequence Hub apps after sequencing completion. |
| • | Standalone mode—Set up a run, following the instructions in NextSeq 1000/2000 Control Software to generate CBCL data. |
Opening the visor during the pre-run check or the run can cause run failure.
Keep hands clear of instrument during visor opening and closing to avoid injury.
| 1. | Configure the run mode, as described in Configure Run Mode. |
| 2. | Select Start. |
| 3. | Enter your BaseSpace Sequence Hub sign-in credentials, and then select Sign In. |
| 4. | If you selected Proactive, Run Monitoring and Storage, select the Workgroup containing your run created in Run Planning in BaseSpace Sequence Hub. |
A workgroup selection is required to avoid errors. Make sure you have selected a workgroup before proceeding.
| 5. | Select Next. |
| 6. | Select your run. |
| 7. | Confirm that the Analysis, Run Length, and Secondary Analysis version matches the correct run. |
Analysis displays Cloud_ to indicate that analysis occurs in BaseSpace Sequence Hub.
| 8. | Select Review. |
| 9. | [Optional] Enter custom read primer and custom index primer locations. |
For information on preparing and adding custom primers, refer to Custom Primers. Make sure to visit the Compatible Products page for your library prep kit to check if Illumina custom primers are necessary.
| 10. | [Optional] Select a custom recipe. For more information, refer to Dark Cycle Sequencing. |
If using the NextSeq 1000/2000 Control Software v1.3 or later and the Illumina Stranded Total RNA Prep with Ribo-Zero Plus kit or the Illumina Stranded mRNA Prep kit, the custom recipe is automatically selected.
| 11. | [Optional] To manually denature and dilute libraries, deselect the Denature and Dilute On Board checkbox. Refer to Standard SBS Manual Denature and Dilute. |
The default selection is configured in the NextSeq 1000/2000 Control Software settings.
| 12. | [Optional] To change the output folder, select the Output Folder field and enter a new location. |
The Output Folder field is autopopulated from your default settings and is required unless Proactive, Run Monitoring and Storage is selected.
If you selected Proactive, Run Monitoring and Storage, Save to BaseSpace Sequence Hub displays Enabled.
If you selected Proactive and Run Monitoring, Save to BaseSpace Sequence Hub displays Disabled.
| 13. | Review your run information, and then select Prep. |
| 1. | Configure the run mode, as described in Configure Run Mode. |
| 2. | Select Start. |
| 3. | If you selected Proactive, Run Monitoring and Storage or Proactive and Run Monitoring, enter your BaseSpace Sequence Hub sign-in credentials, and then select Sign In. |
| 4. | If you selected Proactive, Run Monitoring and Storage, select the BaseSpace Sequence Hub Workgroup to save your run in, and then select Next. |
A workgroup selection is required to avoid errors. Make sure you have selected a workgroup before proceeding.
| 5. | Select Choose... under Start With Sample Sheet, and navigate to the v2 sample sheet on the NextSeq 1000/2000 instrument, portable drive, or mounted network drive. Sample sheet file names cannot contain special characters. |
NextSeq 1000/2000 Control Software v1.3 or later automatically detects the DRAGEN version from the sample sheet and if needed, prompts you to switch versions. The DRAGEN version must be installed on the system. For installation information, refer to Software Updates.
| • | Run Planning Used—Select the .zip folder containing the sample sheet v2 and supporting files if applicable. Otherwise, select the sample sheet v2. |
| • | Run Planning Not Used—Make sure that the secondary analysis supporting file is in the same directory as the sample sheet v2. |
The selected sample sheet must be in v2 format. To create a sample sheet v2, download the generated sample sheet from Run Planning in BaseSpace Sequence Hub or edit a sample sheet v2 template provided on the NextSeq 1000/2000 support page. For more information on sample sheet v2 format and requirements, refer to the Sample Sheet v2 Resource. Make sure any files referenced in the sample sheet are in the same folder as the sample sheet.
| 6. | Select Review. |
| 7. | [Optional] Enter custom read primer and custom index primer locations. |
For information on preparing and adding custom primers, refer to Custom Primers. Make sure to visit the Compatible Products page for your library prep kit to check if Illumina custom primers are necessary.
| 8. | [Optional] Select a custom recipe. For more information, refer to Dark Cycle Sequencing. |
If using the NextSeq 1000/2000 Control Software v1.3 or later and the Illumina Stranded Total RNA Prep with Ribo-Zero Plus kit or the Illumina Stranded mRNA Prep kit, the custom recipe is automatically selected.
| 9. | [Optional] To manually denature and dilute libraries, deselect the Denature and Dilute On Board checkbox. Refer to Standard SBS Manual Denature and Dilute. |
The default selection is configured in the NextSeq 1000/2000 Control Software settings.
| 10. | [Optional] To change the output folder, select the Output Folder field and enter a new location. |
The Output Folder field is autopopulated from your default settings and is required unless Proactive, Run Monitoring and Storage is selected.
If you selected Proactive, Run Monitoring and Storage, Save to BaseSpace Sequence Hub displays Enabled.
If you selected Proactive and Run Monitoring, Save to BaseSpace Sequence Hub displays Disabled.
| 11. | Review your run information, and then select Prep. |
| 1. | Configure the run mode, as described in Configure Run Mode. |
| 2. | Select Start. |
| 3. | If you selected Proactive, Run Monitoring and Storage or Proactive and Run Monitoring, enter your BaseSpace Sequence Hub sign-in credentials, and then select Sign In. |
| 4. | If you selected Proactive, Run Monitoring and Storage, select the BaseSpace Sequence Hub Workgroup to save your run in, and then select Next. |
| 5. | Select Set Up New Run. |
| 6. | In the Run Name field, enter a unique name |
The run name can contain alphanumeric characters, dashes, hyphens, and underscores.
| 7. | For Read Type, select |
| • | Single Read—Perform one read |
| • | Paired End—Perform two reads |
| 8. | Enter the number of cycles Positions where risk of somatic mutations is expected to be elevated. performed in each read: |
There is no maximum number of index cycles, but the sum of the read cycles and index cycles must be less than the number of cycles indicated on the cartridge label plus 38 (or plus 27 if using the 300-cycle cartridge in the NextSeq 1000/2000 P3 Reagents kit). Refer to Supported Number of Cycles.
Read 1—Enter 1–301 cycles.
Index 1—Enter the number of cycles for the Index 1 (i7) primer.
Index 2—Enter the number of cycles for the Index 2 (i5) primer.
Read 2—Enter up to 301 cycles.
| 9. | If you selected Proactive, Run Monitoring and Storage, select Choose... to import a sample sheet. |
NextSeq 1000/2000 Control Software v1.3 or later automatically detects the DRAGEN version from the sample sheet and if needed, prompts you to switch versions. The DRAGEN version must be installed on the system. For installation information, refer to Software Updates.
The selected sample sheet must be in v2 format. To create a sample sheet v2, download the generated sample sheet from Run Planning in BaseSpace Sequence Hub or edit a sample sheet v2 template provided on the NextSeq 1000/2000 support page. For more information on sample sheet v2 format and requirements, refer to the Sample Sheet v2 Resource. Make sure any files referenced in the sample sheet are in the same folder as the sample sheet.
| 10. | [Optional] Enter custom read primer and custom index primer locations. |
For information on preparing and adding custom primers, refer to Custom Primers. Make sure to visit the Compatible Products page for your library prep kit to check if Illumina custom primers are necessary.
| 11. | [Optional] Select a custom recipe. For more information, refer to Dark Cycle Sequencing. |
| 12. | [Optional] To manually denature and dilute libraries, deselect the Denature and Dilute On Board checkbox. Refer to Standard SBS Manual Denature and Dilute. |
The default selection is configured in the NextSeq 1000/2000 Control Software settings.
| 13. | [Optional] To change the output folder, select the Output Folder field and enter a new location. |
The Output Folder field is autopopulated from your default settings and is required unless Proactive, Run Monitoring and Storage is selected.
| 14. | Select Prep. |
| 1. | Make sure that the cartridge was previously thawed and inverted 10 times to mix before loading the flow cell (gray tab removed) and diluted library. |
| 2. | Select Load. |
The NextSeq 1000/2000 Control Software opens the visor and ejects the tray.
| 3. | Place the cartridge onto the tray |
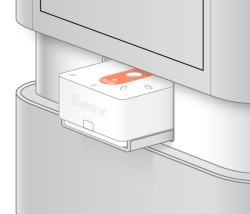
| 4. | Select Close |
The NextSeq 1000/2000 Control Software displays information from the scanned consumables after ~3 minutes.
| 5. | [Optional] Select Eject Cartridge |
The visor opens after 1 minute and ejects the cartridge.
| 6. | Select Sequence. |
Pre-run checks include an instrument check followed by a fluidics check. The fluidics check pierces the cartridge seals, which causes 3-4 popping sounds to emit from the instrument. This sound is expected. The reagent is now passed through the flow cell.
Consumables cannot be reused after the fluidics check starts.
| 1. | Wait |
The run starts automatically after successful completion.
| 2. | If an error occurs |
When a check is in progress, the circle for that check is animated.
| 3. | To troubleshoot recurring errors, refer to Error Message Resolution. |
| 1. | Monitor run progress and metrics |
| • | Estimated run completion—The approximate date and time of run completion. The estimated run completion metric requires 10 previous runs to calculate accurate run completion time. |
| • | Average %Q30—The average percentage of base calls with a Q-score ≥ 30. |
| • | Projected Yield—The expected number of bases called for the run. |
| • | Total Reads PF—The number of paired end (if applicable) clusters passing filtering (in millions). |
| • | Real Time Demux—Status of demultiplexing when initiated at the beginning of Read 2 following completion of Read 1, Index 1, and Index 2 cycles. Status displays Complete even if index cycles are not performed. Not available for Cloud mode runs. |
| • | Real Time Alignment—Status of Read 1 alignment when initiated at the beginning of Read 2 following completion of Read 1, Index 1, and Index 2 cycles. Not available for Cloud mode runs. |
Q30 and yield metrics appear after cycle 26.
| 2. |
To monitor run processes, |
| 3. | To cancel a run, select End Run. Refer to Cancel a Run for more information on canceling runs. |
| 4. | Unload consumables from the instrument. Remove the cartridge from the instrument within 3 days. |
